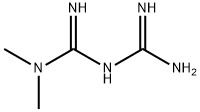
Metformin
- CAS NO.:657-24-9
- Empirical Formula: C4H11N5
- Molecular Weight: 129.16
- MDL number: MFCD00242652
- EINECS: 211-517-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 18:10:20
What is Metformin?
Absorption
Regular tablet absorption
The absolute bioavailability of a metformin 500 mg tablet administered in the fasting state is about 50%-60%. Single-dose clinical studies using oral doses of metformin 500 to 1500 mg and 850 to 2550 mg show that there is a lack of dose proportionality with an increase in metformin dose, attributed to decreased absorption rather than changes in elimination.
At usual clinical doses and dosing schedules of metformin, steady-state plasma concentrations of metformin are achieved within 24-48 hours and are normally measured at <1 μg/mL.
Extended-release tablet absorption
After a single oral dose of metformin extended-release, Cmax is reached with a median value of 7 hours and a range of between 4 and 8 hours. Peak plasma levels are measured to be about 20% lower compared to the same dose of regular metformin, however, the extent of absorption of both forms (as measured by area under the curve - AUC), are similar.
Effect of food
Food reduces the absorption of metformin, as demonstrated by about a 40% lower mean peak plasma concentration (Cmax), a 25% lower area under the plasma concentration versus time curve (AUC), and a 35-minute increase in time to peak plasma concentration (Tmax) after ingestion of an 850 mg tablet of metformin taken with food, compared to the same dose administered during fasting.
Though the extent of metformin absorption (measured by the area under the curve - AUC) from the metformin extended-release tablet is increased by about 50% when given with food, no effect of food on Cmax and Tmax of metformin is observed. High and low-fat meals exert similar effects on the pharmacokinetics of extended-release metformin.
Toxicity
Metformin (hydrochloride) toxicity data:
Oral LD50 (rat): 1 g/kg; Intraperitoneal LD50 (rat): 500 mg/kg; Subcutaneous LD50 (rat): 300 mg/kg; Oral LD50 (mouse): 1450 mg/kg; Intraperitoneal LD50 (mouse): 420 mg/kg; Subcutaneous LD50 (mouse): 225 mg/kg.
A note on lactic acidosis
Metformin decreases liver uptake of lactate, thereby increasing lactate blood levels which may increase the risk of lactic acidosis. There have been reported postmarketing cases of metformin-associated lactic acidosis, including some fatal cases. Such cases had a subtle onset and were accompanied by nonspecific symptoms including malaise, myalgias, abdominal pain, respiratory distress, or increased somnolence. In certain cases, hypotension and resistant bradyarrhythmias have occurred with severe lactic acidosis. Metformin-associated lactic acidosis was characterized by elevated blood lactate concentrations (>5 mmol/L), anion gap acidosis (without evidence of ketonuria or ketonemia), as well as an increased lactate:pyruvate ratio; metformin plasma levels were generally >5 mcg/mL.
Risk factors for metformin-associated lactic acidosis include renal impairment, concomitant use of certain drugs (e.g. carbonic anhydrase inhibitors such as topiramate), age 65 years old or greater, having a radiological study with contrast, surgery and other procedures, hypoxic states (e.g., acute congestive heart failure), excessive alcohol intake, and hepatic impairment.
A note on renal function
In patients with decreased renal function, the plasma and blood half-life of metformin is prolonged and the renal clearance is decreased.
Metformin should be avoided in those with severely compromised renal function (creatinine clearance < 30 ml/min), acute/decompensated heart failure, severe liver disease and for 48 hours after the use of iodinated contrast dyes due to the risk of lactic acidosis. Lower doses should be used in the elderly and those with decreased renal function. Metformin decreases fasting plasma glucose, postprandial blood glucose and glycosolated hemoglobin (HbA1c) levels, which are reflective of the last 8-10 weeks of glucose control. Metformin may also have a positive effect on lipid levels.
A note on hypoglycemia
When used alone, metformin does not cause hypoglycemia, however, it may potentiate the hypoglycemic effects of sulfonylureas and insulin when they are used together.
Use in pregnancy
Available data from post-marketing studies have not indicated a clear association of metformin with major birth defects, miscarriage, or adverse maternal or fetal outcomes when metformin was ingested during pregnancy. Despite this, the abovementioned studies cannot definitively establish the absence of any metformin-associated risk due to methodological limitations, including small sample size and inconsistent study groups.
Use in nursing
A limited number of published studies indicate that metformin is present in human milk. There is insufficient information to confirm the effects of metformin on the nursing infant and no available data on the effects of metformin on the production of milk. The developmental and health benefits of breastfeeding should be considered as well as the mother’s clinical need for metformin and any possible adverse effects on the nursing child.
Description
The study of metformin and its hypoglycemic effects originated from the study of goat’s rue plants, also known as Galega officinalis(French lilac). Goat’s rues are native plants in the Middle East and introduced to Europe later and have been used as forage and ornamental plants throughout the world, including China. As early as in the Middle Ages in Europe, it was found that goat’s rues could ease polyuria, which is one of the typical symptoms of diabetes. While goat’s rues were used to treat a variety of other diseases in the Middle Ages, it was found to cause poisoning symptoms in livestock. Goat’s rues are still used as medical plants at present, mainly for diabetes, diuretic, hepatoprotection, aiding in digestion and promoting lactation, etc. In China, goat’s rues were recorded first in the dictionary of Chinese seed plants and mainly used for the treatment of diabetes. However, because of high toxicity, it is rarely used in traditional Chinese medicines at present.
Physical properties
Appearance: white crystalline or crystalline powder, odorless. Solubility: freely soluble in water, soluble in methanol, slightly soluble in ethanol, and insoluble in chloroform or ether. Melting point: 223–226°C.
Originator
Diabetex ,Germania
History
Metformin is a biguanide compound which originated from the extraction of goat’s
rue plants. The structure of metformin was identified by British scholars in the early
1920s. In 1922, Werner and Bell et?al. first synthesized metformin in 31 institutes in
Dublin, Ireland. In 1929, Slotta and Tschesche found metformin’s hypoglycemic action. However, because of other potent antidiabetic drugs such as insulin
which were widely used in clinical practice, the pharmacological effects of metformin didn’t receive much attention.
Until the 1950s, a French diabetic scientist Jean Sterne found the hypoglycemic
effect of metformin through the study of galegine. Then the drug was used in diabetic patients for the first time, and the results were published in 1957. UKPDS, which began from 1977 and ended in 1997 and
was then followed up for 10?years, is the longest in the history of clinical trials and
has a significant impact on practice and guidelines for prevention and treatment of
diabetes mellitus. In this trial, metformin was found to reduce the risk of diabetic
complications by 32%. In addition, it was proved for the first time that metformin
can reduce blood glucose and protect against cardiovascular function, especially in
obese patients. In 1994, metformin was approved by the US FDA for type 2
diabetes treatment. Currently, metformin has become the world’s most widely used
antidiabetic drug.
Aiming at improving the stability of the absorption of metformin, chemists have
also carried out a series of structural renovation and modification. Metformin activates with carbonyl, esters, chlorides, and aldehydes to form triazine compounds,
with 1,3-diketone to produce pyrimidine compounds, and with disulfides to produce
C-S coupling products, etc.
The Uses of Metformin
non-insulin dependent diabetes mellitus
Background
Metformin is a biguanide antihyperglycemic agent and first-line pharmacotherapy used in the management of type II diabetes.
Metformin is considered an antihyperglycemic drug because it lowers blood glucose concentrations in type II diabetes without causing hypoglycemia. It is commonly described as an "insulin sensitizer", leading to a decrease in insulin resistance and a clinically significant reduction of plasma fasting insulin levels. Another well-known benefit of this drug is modest weight loss, making it an effective choice for obese patients type II diabetes.
Metformin was first approved in Canada in 1972, and received subsequent FDA approval in the US in 1995.
Indications
Metformin immediate-release formulations
Metformin is indicated as an adjunct to diet and exercise to improve glycemic control in adults and pediatric patients ≥10 years old with type 2 diabetes mellitus.
Metformin extended-release tablet (XR)
The extended-release formulation of metformin is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. Safety in children has not been determined to this date.
Metformin combination products
Metformin is a component of a variety of combination products with other anti-diabetic agents. It is indicated, along with diet and exercise, to improve glycemic control in adult patients with type 2 diabetes mellitus in combination with DPP-4 inhibitors (sitagliptin, linagliptin, alogliptin, or saxagliptin), in combination with SGLT2 inhibitors (canagliflozin, empagliflozin, ertugliflozin, or dapagliflozin), or in combination with pioglitazone.
Indications
Metformin (Glucophage) was used in Europe for many years before it was approved for use in the United States in 1995. Metformin is the only approved biguanide for the treatment of patients with NIDDM that are refractory to dietary management alone. Metformin does not affect insulin secretion but requires the presence of insulin to be effective. The exact mechanism of metformin’s action is not clear, but it does decrease hepatic glucose production and increase peripheral glucose uptake. When used as monotherapy, metformin rarely causes hypoglycemia.
Definition
ChEBI: Metformin is a member of the class of guanidines that is biguanide the carrying two methyl substituents at position 1. It has a role as a hypoglycemic agent, a xenobiotic, an environmental contaminant and a geroprotector. It is functionally related to a biguanide. It is a conjugate base of a metformin(1+).
Manufacturing Process
The boiling mixture of 1,000 L xylene, 450 kg dimethylamine and 840 kg dicyanamide was added 365 kg hydrogene chloride. Yield of biguanide, 1,1- dimethyl-, hydrochloride 1,588 kg (96%). Biguanide, 1,1-dimethyl-, hydrochloride may be recrystallysed from methanol.
Therapeutic Function
Oral hypoglycemic
Biological Functions
Metformin can lower free fatty acid concentrations by 10 to 30%. This antilipolytic effect may help to explain the reduction in gluconeogenesis through reduced levels of available substrate (65). When given as a monotherapy, metformin treatment does not lead to hypoglycemia, so it is better described as an antihyperglycemic agent rather than a hypoglycemic agent.
General Description
Metformin, N,N-dimethylimidodicarbonimidicdiamide hydrochloride (Glucophage), is a bisguanidine.This class of agents is capable of reducing sugar absorptionfrom the gastrointestinal tract. Also, they can decrease gluconeogenesiswhile increasing glucose uptake by muscles andfat cells. These effects, in turn, lead to lower blood glucoselevels. Unlike the sulfonylureas, these are not hypoglycemicagents but rather can act as antihyperglycemics. This differencein nomenclature is caused by the inability of these agentsto stimulate the release of insulin from the pancreas. Often,metformin is coadministered with the nonsulfonylureas to improvethe efficacy of those agents.
Mechanism of action
The mechanism of action of biguanides is still not fully understood. Three major tissues have been identified as pharmacological sites of action: (1) the small intestinal wall, (2) the liver, and (3) peripheral tissues, mainly the skeletal muscle: 1. For the small intestine an inhibition of glucose absorption was described, however, this is, at least for metformin, of minor significance and not important for the blood glucose lowering effect. However, the intestinal glucose metabolization to lactate is stimulated and reduces the postprandial uptake of glucose by the liver. 2. Numerous studies have shown that biguanides inhibit hepatic gluconeogenesis and this may contribute to the blood glucose lowering effect, particularly in the fasting state. Again, metformin has probably less impact on gluconeogenesis than phenformin and buformin. 3. In the peripheral tissues, metformin increases the glucose disposal and utilization particularly in the skeletal muscle, which is probably the major contribution to the blood glucose lowering activity. In vitro studies using cell cultures have shown that metformin potentiates insulin action. In vivo studies in animals and diabetic patients have demonstrated that metformin reduces insulin resistance, at least in obese individuals.
Pharmacokinetics
General effects
Insulin is an important hormone that regulates blood glucose levels. Type II diabetes is characterized by a decrease in sensitivity to insulin, resulting in elevations in blood glucose when the pancreas can no longer compensate. In patients diagnosed with type 2 diabetes, insulin is unable to exert adequate effects on tissues and cells (i.e. insulin resistance) and insulin deficiency may also be present.
Metformin reduces hepatic production of glucose, decreases the intestinal absorption of glucose, and enhances insulin sensitivity by increasing both peripheral glucose uptake and utilization. In contrast with drugs of the sulfonylurea class, which lead to hyperinsulinemia, the secretion of insulin is unchanged with metformin use.
Effect on fasting plasma glucose (FPG) and Glycosylated hemoglobin (HbA1c)
HbA1c is an important periodic measure of glycemic control used to monitor diabetic patients. Fasting plasma glucose is also a useful and important measure of glycemic control. In a 29-week clinical trial of subjects diagnosed with type II diabetes, metformin decreased the fasting plasma glucose levels by an average of 59 mg/dL from baseline, compared to an average increase of 6.3 mg/dL from baseline in subjects taking a placebo. Glycosylated hemoglobin (HbA1c) was decreased by about 1.4% in subjects receiving metformin, and increased by 0.4% in subjects receiving placebo only.
Pharmacology
As a traditional antidiabetic drug, metformin can reduce the levels of blood glucose and lipid, as well as regulating cell growth, anti-inflammation, antiaging, etc. The main pharmacological mechanisms are inhibition of hepatic gluconeogenesis, the activation of AMP-activated protein kinase (AMPK), and the regulation of mitochondrial function.
Improvement of Insulin Resistance and Decrease of Blood Glucose Levels
The main pharmacological effects of biguanide drugs are to reduce blood glucose output and improve peripheral insulin resistance. Metformin inhibits hyperglycemia mainly by inhibiting hepatic glucose production (hepatic gluconeogenesis), increasing muscle glucose uptake.
Regulation of Lipid Metabolism and Reducing Body Weight
Metformin can reduce the blood triglycerides in circulation, improve liver steatosis, promote the oxidation of brown adipose tissue and VLDL-fatty acid triglyceride uptake, and inhibit fat formation; the process may be related with the activation of AMPK. AMPK can phosphorylate acetyl coenzyme A carboxylase (ACC) and inhibit the conversion of acetyl coenzyme A into malonyl-CoA. Malonyl-CoA is a precursor of fatty acid production and is an allosteric inhibitor of fatty acid transporting to mitochondria.
Prevention and Treatment of Tumors
Epidemiological studies have shown that metformin reduced the risk of multiple types of tumors in type 2 diabetes and nondiabetes patients and reduced tumor-related mortality. Metformin also has a therapeutic effect on various tumors. The antitumor effect of metformin may be through the reduction of serum insulin and insulin-like growth factor-1 (IGF-1) levels or activation of LKB1/AMPK, thereby blocking the mammalian target of rapamycin-sensitive complex 1 (mTORC1) signaling pathway.
Antiaging Antiaging effect is a major discovery in the new role of metformin. FDA approved the phase 4 of clinical trial of metformin for antiaging effect, and the trial is ongoing. The study suggests that the antiaging effect of metformin is closely related to the mitochondria, through the regulation of mitochondrial function to activate AMPK and inhibit mTOR, thereby reducing energy consumption. Furthermore, metformin can regulate oxidative stress, reduce tissue inflammation, and reduce the growth factor level and cell proliferation. The above effects and the combinational effect result in the improvement of health and achieving longevity.
Other Effects Studies have shown that metformin helps to prevent type 2 diabetes. For young people with a high body mass index, metformin is more effective than lifestyle control.
In addition, metformin can improve and prevent vascular disease, improve mitochondrial function, and serve as anti-inflammatory agent and antioxidant.
Metformin is hydrophilic and distributed in tissues in an active manner. After
oral administration, metformin is absorbed quickly and rapidly distributed into various tissues, mainly in the liver, gastrointestinal tract, and kidney.
Clinical Use
Metformin works best in patients with significant hyperglycemia and is often considered first-line therapy in the treatment of mild to moderate type II overweight diabetics who demonstrate insulin resistance. The United Kingdom Prospective Diabetes Study demonstrated a marked reduction in cardiovascular comorbidities and diabetic complications in metformintreated individuals. Metformin has also been used to treat hirsutism in individuals with polycystic ovarian syndrome and may enhance fertility in these women, perhaps by decreasing androgen levels and enhancing insulin sensitivity.
Safety Profile
Poison by subcutaneous and intraperitoneal routes. Mildly toxic by parenteral route. Experimental teratogenic effects. Mutation data reported. When heated to decomposition it emits toxic fumes of NOx
Metabolism
Intravenous studies using a single dose of metformin in normal subjects show that metformin is excreted as unchanged drug in the urine and does not undergo hepatic metabolism (no metabolites have been identified in humans) or biliary excretion.
Metabolism
Metformin is quickly absorbed from the small intestine. Bioavailability is from 50 to 60%, and the drug is not protein bound. Peak plasma concentrations occur at approximately 2 hours. The drug is widely distributed in the body and accumulates in the wall of the small intestine. This depot of drug serves to maintain plasma concentrations. Metformin is excreted in the urine, via tubular excretion, as unmetabolized drug with a half-life of approximately 2 to 5 hours; therefore, renal impairment as well as hepatic disease are contraindications for the drug.
Properties of Metformin
| Melting point: | 199-200 °C |
| Boiling point: | 229.23°C (rough estimate) |
| Density | 1.0743 (rough estimate) |
| refractive index | 1.5760 (estimate) |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| solubility | Acetonitrile (Slightly), Aqueous Acid (Slightly), Dichloromethane (Slightly), DM |
| form | Solid |
| pka | pKa 2.8(H2O,t =32) (Uncertain) |
| color | White to Light Brown |
| Water Solubility | Water: 50 mg/mL (387.12 mM) |
| InChI | InChI=1S/C4H11N5/c1-9(2)4(7)8-3(5)6/h1-2H3,(H5,5,6,7,8) |
| CAS DataBase Reference | 657-24-9(CAS DataBase Reference) |
| EPA Substance Registry System | Imidodicarbonimidic diamide, N,N-dimethyl- (657-24-9) |
Safety information for Metformin
Computed Descriptors for Metformin
| InChIKey | XZWYZXLIPXDOLR-UHFFFAOYSA-N |
| SMILES | C(=N)(N(C)C)NC(=N)N |
Metformin manufacturer
Clickchem Research LLP
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 657-24-9 99%View Details
657-24-9 99%View Details
657-24-9 -
 Metformin 98%View Details
Metformin 98%View Details
657-24-9 -
 Metformin 657-24-9 98%View Details
Metformin 657-24-9 98%View Details
657-24-9 -
 657-24-9 Metformin 99%View Details
657-24-9 Metformin 99%View Details
657-24-9 -
 657-24-9 98%View Details
657-24-9 98%View Details
657-24-9 -
 Metformin 98%View Details
Metformin 98%View Details
657-24-9 -
 657-24-9 98%View Details
657-24-9 98%View Details
657-24-9 -
 Metformin HCL IP / BP / USPView Details
Metformin HCL IP / BP / USPView Details
1115-70-4