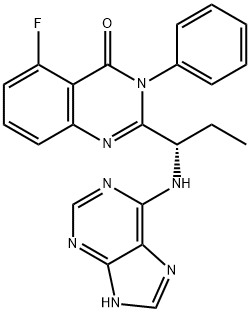
Ibrutinib
- CAS NO.:936563-96-1
- Empirical Formula: C25H24N6O2
- Molecular Weight: 440.5
- MDL number: MFCD20261150
- EINECS: 805-642-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 16:58:18

What is Ibrutinib?
Absorption
Ibrutinib is rapidly absorbed after oral administration and it presents a Cmax, tmax and AUC of approximately 35 ng/ml, 1-2 hour and 953 mg.h/ml respectively.
Toxicity
Ibrutinib was not showed to present a mutagenic potential in bacterial assays, nor clastogenic in chromosome aberration assays in mammalian cells or in bone marrow micronucleus assays in mice. Carcinogenicity or effects on fertility have not been determined.
Description
Ibrutinib is a cancer drug that targets B-cell malignancies such as certain leukemias and lymphomas. Its design and synthesis were reported in 2007 by Z. Pan and co-workers at Celera Genomics (South San Francisco, CA, and Rockville, MD). By that time, Pharmacyclics (Sunnyvale, CA) had acquired ibrutinib and related compounds.
Ibrutinib was approved by the US Food and Drug Administration in 2013 for treating mantle cell lymphoma and in 2014 for treating chronic lymphocytic leukemia. The lymphoma application was submitted in June 2013 and, under FDA’s breakthrough therapy program, it was approved 4 months later.
The 2013 approval was also noteworthy because?Pharmacyclics had sufficient quantities of the drug to make it commercially available?immediately. A key factor was Pharmacyclics’ long partnership with Lonza, a Switzerland-based multinational company with custom manufacturing facilities in the United States.
Description
Ibrutinib (CAS: 936563-96-1) is a first-in-class,potent, orally administered covalently-binding inhibitor of BTK. In November 2013, the US FDA approved ibrutinib (also referred to as PCI-32765), for the treatment of patients with mantle cell lymphoma (MCL) who had received at least one prior therapy. Ibrutinib is a potent inhibitor of BTK that binds covalently to Cys-481 in the active site of BTK, resulting in inhibition of kinase activity. Ibrutinib does have significant activity against 19 other kinases, including seven with a cognate cysteine residue. These include BLK, BMX, ITK, TEC, EGFR, ERBB2, and JAK3.
Originator
Celera/Pharmacyclics (United States)
The Uses of Ibrutinib
Ibrutinib is an irreversible Bruton′s tyrosine kinase inhibitor that selectively blocks B cell activation.
Background
Ibrutinib is a small molecule that acts as an irreversible potent inhibitor of Burton's tyrosine kinase. It is designated as a targeted covalent drug and presented as a promising activity in B-cell malignancies in clinical trials. Ibrutinib was developed by Pharmacyclics Inc and was first approved by the FDA in November 2013 for the treatment of mantle cell lymphoma (MCL) under accelerated approval; however, in April 2023, the drug manufacturer withdrew the accelerated approvals for ibrutinib in the US.
Ibrutinib was approved by the EMA in October 2014 and by Health Canada in November 2014. It is currently approved for the treatment of various conditions, such as chronic lymphocytic leukemia (CLL), Waldenstr?m's Macroglobulinemia, and chronic graft versus host disease (cGVHD) in August 2017. Notably, ibrutinib became the first FDA-approved cGVHD treatment for children in August 2017.
Indications
Ibrutinib is indicated for the treatment of the following conditions.
Chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL)
Waldenstr?m's macroglobulinemia
Chronic graft-versus-host disease (cGVHD)
Mantle cell lymphoma (MCL)
Marginal zone lymphoma (MZL)
Definition
ChEBI: Ibrutinib is a member of the class of acrylamides that is (3R)-3-[4-amino-3-(4-phenoxyphenyl)pyrazolo[3,4-d]pyrimidin-1-yl]piperidine in which the piperidine nitrogen is replaced by an acryloyl group. A selective and covalent inhibitor of the enzyme Bruton's tyrosine kinase, it is used for treatment of B-cell malignancies. It has a role as an EC 2.7.10.2 (non-specific protein-tyrosine kinase) inhibitor and an antineoplastic agent. It is a pyrazolopyrimidine, an aromatic amine, an aromatic ether, a member of acrylamides, a N-acylpiperidine and a tertiary carboxamide.
Indications
Ibrutinib is a non-receptor Bruton's tyrosine kinase inhibitor approved for the treatment of relapsed chronic lymphocytic leukemia(CLL), mantle cell lymphoma (MCL) and WaldenstrÖm's Macroglobulinemia (WM).
brand name
Imbruvica
General Description
Class: non-receptor tyrosine kinase
Treatment: CLL, MCL
Oral bioavailability = 4% (fasted), 8% (fed)
Elimination half-life = 3.2 h
Protein binding = 97.3%
Pharmacokinetics
In vitro studies have shown an induction of CLL cell apoptosis even in presence of prosurvival factors. It has also been reported an inhibition of CLL cell survival and proliferation as well as an impaired in cell migration and a reduction in the secretion of chemokines such as CCL3 and CCL4. The latter effect has been shown to produce regression in xenograft mouse models.
Clinical studies for relapsed/refractory CLL in phase I and II showed an approximate 71% of overall response rate.. In the case of relapsed/refractory mantle cell lymphoma, approximately 70% of the tested patients presented a partial or complete response.. In clinical trials for relapsed/refractory diffuse large B-cell lymphoma, a partial response was found in between 15-20% of the patients studied; while for patients with relapsed/refractory Waldenstrom's macroglobulinemia, a partial response was observed in over 75% of the patients tested. Finally, for patients with relapsed/refractory follicular lymphoma, a partial to complete response was obtained in approximately 54% of the patients.
Side Effects
The more common side effects of Ibrutinib are diarrhoea and bleeding. Therefore, you should drink plenty of water during treatment, in order to reduce the risk of dehydration using diarrhoea. Bleeding problems can be serious and may even lead to death. This may be manifested by blood or black stools (that look like tar); pink or brown urine; accidental bleeding, or severe or uncontrollable bleeding; spitting up blood or vomit that looks like coffee grounds; coughing up blood; bruising or small red or purple spots on the skin; dizziness; confusing changes in speech; or a headache that lasts for a long time or is severe. Prompt medical attention is needed if a serious condition develops.
Other possible side effects are: increased risk of infection, fever, chills, weakness, confusion, or other signs or symptoms of infection; increased risk of heart disease, rapid and irregular heartbeat; dizziness; lightheadedness; shortness of breath; swelling of the feet, ankles, or legs; discomfort in the chest; or feeling lightheaded. High blood pressure, reduced blood counts, second primary cancer and tumour lysis syndrome (TLS). Different disease treatments exhibit different adverse effects, so the above side effects may not occur in full.
Synthesis
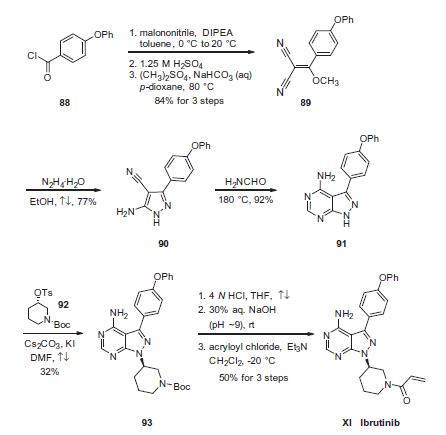
Condensation of commercially available 4-phenoxybenzoyl chloride (88) with malononitrile followed by acidic quench and O-methylation with dimethyl sulfate furnished vinyl dinitrile 89 in 84% yield over the three-step sequence. Next, treatment with hydrazine hydrate in refluxing ethanol secured aminopyrazole 90 and this was followed by treatment with neat formamide at elevated temperature to furnish pyrimidopyrazole 91 in excellent conversion. Selective alkylation of the pyrazole nitrogen with commercially- available (S)-piperidinyl tosylate (92) proceeded in 32% yield. Finally, liberation of the amide followed by pH adjustment and amide bond formation with acrolyl chloride furnished ibrutinib (XI) in 50% over the three-step sequence.
Drug interactions
Potentially hazardous interactions with other drugs
Anti-arrhythmics: concentration possibly increased
by amiodarone and dronedarone - avoid or reduce
dose of ibrutinib.
Antibacterials: concentration possibly increased
by ciprofloxacin, clarithromycin, erythromycin and
telithromycin - avoid or reduce dose of ibrutinib;
concentration reduced by rifampicin - avoid.
Antidepressants: concentration possibly reduced by
St John’s wort - avoid.
Antiepileptics: concentration possibly reduced by
carbamazepine, fosphenytoin, phenobarbital and
phenytoin - avoid.
Antifungals: concentration possibly increased
by fluconazole, itraconazole, ketoconazole and
voriconazole - avoid or reduce dose of ibrutinib
.
Antipsychotics: avoid with clozapine, increased risk
of agranulocytosis.
Antivirals: concentration possibly increased by
atazanavir, darunavir, fosamprenavir, indinavir,
ritonavir and saquinavir - avoid or reduce dose of
ibrutinib.
Aprepitant: concentration possibly increased - avoid
or reduce dose of ibrutinib.
Calcium channel blockers: concentration possibly
increased by diltiazem or verapamil - avoid or reduce
dose of ibrutinib.
Cobicistat: concentration possibly increased, avoid or
reduce dose of ibrutinib.
Cytotoxics: concentration possibly increased by
crizotinib - avoid or reduce dose of ibrutinib;
concentration possibly increased by imatinib -
reduce dose of ibrutinib.
Grapefruit juice and Seville oranges: avoid.
Metabolism
Three metabolic pathways have been identified according to the possible metabolites. These pathways are the hydroxylation of the phenyl group (M35), the opening of the piperidine with a reduction of the primary alcohol (M34) and the oxidation to a carboxylic acid and epoxidation of the ethylene followed by a hydrolysis to the formation of dihydrodiol (PCI-45227). The latter metabolite presents also 15 times lower inhibitory activity against BTK. The metabolism of ibrutinib is mainly performed by CYP3A5 and CYP3A4. and in a minor extent it is seen to be performed by CYP2D6.
Metabolism
Ibrutinib is metabolised primarily by CYP3A4 to
produce a dihydrodiol metabolite with an inhibitory
activity towards BTK approximately 15 times lower
than that of ibrutinib. Involvement of CYP2D6 in the
metabolism of ibrutinib appears to be minimal.
After a single oral administration of radiolabeled
[14C]-ibrutinib in healthy subjects, approximately 90%
of radioactivity was excreted within 168 hours, with the
majority (80%) excreted in the faeces and <10% in the
urine. Unchanged ibrutinib accounted for approximately
1% of the radiolabeled excretion product in faeces and
none in urine.
Storage
+4°C
Mode of action
Ibrutinib is the second oral agent approved for the treatment of MCL. It works by irreversibly inhibiting Bruton’s tyrosine kinase (Btk) leading to the inhibition of B-cell receptor signaling and resulting in the reduction of malignant B-cell proliferation and induction of cell death. Btk plays an important role in the differentiation, development, proliferation, and survival of B cells via activation of cell-cycle regulators and regulating the expression of pro- and antiapoptotic proteins. Aberrant Btk activity results in a variety of B-cell malignancies including MCL. Ibrutinib inhibits Btk by irreversibly binding to cysteine-481 in the active site thereby inhibiting phosphorylation of tyrosine-223 and affecting downstream B-cell signaling pathways.
References
1) Honigberg?et al.?(2010),?The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy;?Proc. Natl. Acad. Sci. USA?107?13075 DOI:10.1073/pnas.1004594107
2) Ponader?et al.?(2012),?The Bruton tyrosine kinase Inhibitor PCI-32765 thwarts chronic lymphocytic leukemia cell survival and tissue homing in vitro and in vivo;?Blood?119?1182
DOI:10.1182/blood-2011-10-386417
3) De Rooij?et al.?(2012),?The clinically active BTK inhibitor PCI-32765 targets B-cell receptor- and chemokine-controlled adhesion and migration in chronic lymphocytic leukemia;?Blood?119?2590
DOI:10.1182/blood-2011-11-390989
4) Pavlasoca?et al.?(2016),?Ibrutinib inhibits CD20 upregulation on CLL B cells mediated by the CXCR4/SDF-1 axis;?Blood?128?1609 DOI:10.1182/blood-2016-04-709519
5) Sagiv-Barfi?et al.?(2015),?Therapeutic antitumor immunity by checkpoint blockade is enhanced by ibrutinib, an inhibitor of both BTK and ITK; Proc. Natl. Acad. Sci. USA?112?E966 DOI:10.1073/pnas.1500712112
6) Weber?et al.?(2017),?Bruton's Tyrosine Kinase: An Emerging Key Player in Innate Immunity,?Front. Immunol.?8?1454 DOI:10.3389/fimmu.2017.01454
Properties of Ibrutinib
| Melting point: | 153-158°C |
| Boiling point: | 715.0±60.0 °C(Predicted) |
| Density | 1.34 |
| storage temp. | -20°C |
| solubility | Soluble in DMSO ( up to at least 25 mg/ml) |
| form | solid |
| pka | 4.09±0.30(Predicted) |
| color | White or off-white |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 3 months. |
| CAS DataBase Reference | 936563-96-1 |
Safety information for Ibrutinib
Computed Descriptors for Ibrutinib
| InChIKey | XYFPWWZEPKGCCK-GOSISDBHSA-N |
| SMILES | C(N1CCC[C@@H](N2C3C(C(C4=CC=C(OC5=CC=CC=C5)C=C4)=N2)=C(N)N=CN=3)C1)(=O)C=C |
Ibrutinib manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 936563-96-1 98%View Details
936563-96-1 98%View Details
936563-96-1 -
 Ibrutinib 936563-96-1 99%View Details
Ibrutinib 936563-96-1 99%View Details
936563-96-1 -
 Ibrutinib 99%View Details
Ibrutinib 99%View Details
936563-96-1 -
 936563-96-1 Ibrutinib 98%View Details
936563-96-1 Ibrutinib 98%View Details
936563-96-1 -
 936563-96-1 99%View Details
936563-96-1 99%View Details
936563-96-1 -
 Ibrutinib 99%View Details
Ibrutinib 99%View Details -
 Ibrutinib 98% CAS 936563-96-1View Details
Ibrutinib 98% CAS 936563-96-1View Details
936563-96-1 -
 Ibrutinib (PCI-32765) 99% (HPLC) CAS 936563-96-1View Details
Ibrutinib (PCI-32765) 99% (HPLC) CAS 936563-96-1View Details
936563-96-1