Troglitazone
Synonym(s):(±)-5-[4-[(6-Hydroxy-2,5,7,8-tetramethylchroman-2-yl)methoxy]benzyl]-2,4-thiazolidinedione;CS-045;Troglitazone - CAS 97322-87-7 - Calbiochem
- CAS NO.:97322-87-7
- Empirical Formula: C24H27NO5S
- Molecular Weight: 441.54
- MDL number: MFCD00878416
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 15:53:33
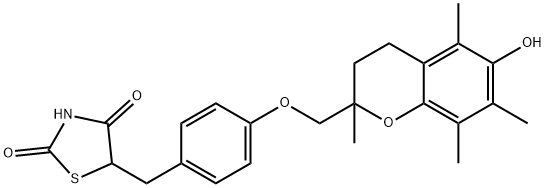
What is Troglitazone?
Absorption
Absorbed rapidly. Food increases the extent of absorption by 30% to 85%.
Description
Rezulin was launched in Japan, the UK (subseqently withdrawn) and the US for treatment of type Ⅱ diabetes. Two approaches, four steps and six steps, converge on 6-acetoxy-2-(4-aminophenoxymethyl)-2,5,7,8-tetramethylchroman which can be elaborated in two steps to Rezulin. It is the first of a new class of thiazolidenediones for NIDDM that reduces glucose concentrations without effecting insulin secretion. It binds to peroxisome proliferator-activated receptor gamma (PPARγ) thus activating this nuclear receptor which then influences carbohydrate metabolism. This is accomplished by increasing insulin sensitivity by upregulating glucose transporter (Glut1 and/or Glut4) expression without affecting the number or affinity of insulin receptors. There is also an increase in hepatic glycogen synthase activity which enhances glucose utilization and a reduction in hepatic gluconeogenesis by inhibiting fructose-1 ,&bisphosphatase. Pancreatic islet cell destruction is prevented. It reduces serum triglycerides because PPARγ causes fibroblasts to differentiate into adipocytes and does not activate RARα. It has a half-life of 9 h and is metabolized into three compounds having no activity.
Chemical properties
Crystalline Solid
Originator
Sankyo (Japan)
History
Troglitazone (Sankyo) was firstly approved 1995 in Japan and 1997 in USA and UK. It was launched in these countries in 1997. The recommended starting dose was 400 mg/d and the highest dose 600 mg. After being marketed, the daily practice revealed adverse events in the liver with cases of fatal liver failure. This lead to withdrawal of troglitazone from the market in the UK already after two months, in USA and Japan in the year 2000.
The Uses of Troglitazone
Troglitazone is an an oral hypoglycemic agent which improves insulin sensitivity and decreases hepatic glucose production. Antidiabetic.
The Uses of Troglitazone
antidiabetic;peroxisome proliferator-activated receptors activator
The Uses of Troglitazone
Troglitazone is a TZD which was approved for the treatment of insulin resistance and hyperglycemia in Type II diabetes, under the trade name Resulin?, but was withdrawn from the market due to hepatotoxicity. Troglitazone is a potent and selective PPARγ agonist. The EC50 values for transactivation of human and mouse PPARγ in a cell-based assay are 0.55 and 0.78 μM, respectively. In the same assay system, no activation of PPARα and PPARδ was observed at concentrations up to 10 μM. Troglitazone binds to the PPARγ ligand-binding domain (LBD) but fails to induce interaction of the PPARγ LBD with the transcriptional coactivators SRC-1, TIF2, AIB1, p300, or TRAP220. Troglitazone also induces cell cycle arrest and apoptosis in several cancer cell lines with an EC50 of 10 μM.
Background
Troglitazone was withdrawn in 2000 due to risk of hepatotoxicity. It was superseded by pioglitazone and rosiglitazone.
Indications
For the treatment of Type II diabetes mellitus. It is used alone or in combination with a sulfonylurea, metformin, or insulin as an adjunct to diet and exercise.
What are the applications of Application
Troglitazone is a potent antiproliferative, apoptosis inducer, antioxidant, and antiinflammatory agent
Definition
ChEBI: Troglitazone is a member of chromanes and a thiazolidinone. It has a role as a hypoglycemic agent, an antioxidant, a vasodilator agent, an anticonvulsant, an anticoagulant, a platelet aggregation inhibitor, an antineoplastic agent, an EC 6.2.1.3 (long-chain-fatty-acid--CoA ligase) inhibitor and a ferroptosis inhibitor.
Manufacturing Process
A mixture of 70 g of ethyl-3-[4-(6-acetoxy-2,5,7,8-tetramethylchroman-2- ylmethoxy)phenyl]-2-chloropropionate, 13.12 g of thiourea and 80.2 ml of sulpholane was reacted for 80 min, under a nitrogen stream at 115°-120°C. Subsequently, a 656.2 ml acetic acid, 218.7 ml conc. hydrochloric acid and 109.4 ml water was added to this and the resulting mixture was further heated for 12 h at 85°-90°C. The reaction mixture was cooled to room temperature and 196.8 g of sodium bicarbonate was added and once the evolution of carbondioxide had ceased, the solvent was distilled off applying high vacuum. A 10:1 by volume mixture of benzene and ethyl acetate was added to the residue and the crude product was washed with a mixture of equal volumes of a saturated aq. sodium bicarbonate solution water. The organic layer was dried over anhydrous sodium sulphate and the solvent was distilled off. The resulting crude product was quickly eluted from a silica gel column with 50% ethylacetate-hexane to furnish 40 g of the required 5-{4- (6-hydroxy-2,5,7,8-tetramethylchroman-2-yl-methoxy)benzyl)thiazolidine-2,4- dione (Troglitazone) with a HPLC purity of about 67-70%. The elution of column was continued further to yield 5-[4-(6-hydroxy-2,5,7,8- tetramethylchroman-2-yl-methoxy)benzyl]-2-iminothiazolidine-4-one with HPLC purity of about 70%.
brand name
Prelay (Sankyo); Rezulin (Pfizer);Ronglitazone.
Therapeutic Function
Hypoglycemic
General Description
A α-tocopherol (vitamin E) moiety containing thiazolidinedione class of insulin-sensitizer that acts as an activator of peroxisome proliferator-activated receptors γ (PPARγ). Also exhibits anti-proliferative and anti-inflammatory properties. Shown to induce cell cycle arrest and apoptosis in human hepatoma cells. Inhibits vascular smooth muscle cell (VSMC) migration by rapidly activating MEK/ERK pathway leading to the induction of c-fos and Ets-1 expression. Blocks PDGF-induced cell proliferation of pancreatic stellate and accelerates p21Cip1 turnover by inhibiting PKCδ signaling in VSMCs. Exerts positive inotropic effect via Ca2+ sensitization in the sarcoplasmic reticulum and inhibits Na+/H+ exchange activity and proliferation of macrovascular endothelial cells.
Biological Activity
Selective PPAR γ receptor agonist (EC 50 values are 780 and 555 nM at murine and human PPAR γ receptors respectively). Displays no activity at PPAR α or PPAR δ receptors. Antidiabetic agent; exerts potent glucose-lowering effects in insulin-resistant diabetic mice. Displays anti-invasive effect on human breast cancer cells; reduces migration, adhesion and spreading on fibronectin-coated plates. Also inhibits lamellipodia formation and actin polymerization.
Biochem/physiol Actions
Primary TargetPeroxisome proliferator-activated receptors γ (PPARγ)
Pharmacokinetics
Troglitazone is an oral antihyperglycemic agent which acts primarily by decreasing insulin resistance. Troglitazone is used in the management of type II diabetes (noninsulin-dependent diabetes mellitus (NIDDM) also known as adult-onset diabetes). It improves sensitivity to insulin in muscle and adipose tissue and inhibits hepatic gluconeogenesis. Troglitazone is not chemically or functionally related to either the sulfonylureas, the biguanides, or the g-glucosidase inhibitors. Troglitazone may be used concomitantly with a sulfonylurea or insulin to improve glycemic control.
Pharmacology
Depending on the animal model, after repeated dosing troglitazone lowered hyperglycemia as well as levels of free fatty acids, triglycerides, and insulin at daily doses of 50 mg/kg p.o. or slightly lower.
in vitro
troglitazone pparγ ligands showed potent inhibitory effect on proliferation, and could induce rcc cell apoptosis, suggesting that the pparγ ligands have potential antitumor effects on renal carcinoma cells [1].
in vivo
troglitazone increased ec proliferation in brown and white adipose tissue and liver in mice at sarcomagenic doses (400 and 800 mg/kg) after four weeks of treatment [2].
Metabolism
A sulfate conjugate metabolite (Metabolite 1) and a quinone metabolite (Metabolite 3) have been detected in the plasma of healthy males. A glucuronide conjugate (Metabolite 2) has been detected in the urine and also in negligible amounts in the plasma. In healthy volunteers and in patients with type 2 diabetes, the steady-state concentration of Metabolite 1 is six to seven times that of troglitazone and Metabolite 3. In in vivo drug interaction studies, troglitazone has been shown to induce cytochrome P450 CYP3A4 at clinically relevant doses.
storage
Store at -20°C
References
γ1) Kodera et al. (2000) Ligand type-specific interactions of peroxisome proliferator-activated receptor gamma with transcriptional coactivators; J. Biol. Chem., 275 33201 2) Tchoukalova et al. (2000) Enhancing effect of troglitazone on porcine adipocyte differentiation in primary culture: a comparison with dexamethasone; Obes. Res., 8 664 3) Tsubouchi et al. (2000) Inhibition of human lung cancer cell growth by the peroxisome proliferator-activated receptor-gamma agonists through induction of apoptosis; Biochem. Biophys. Res. Commun.270 4) Wyttenbach & Kuentz (2017),?Glass-forming ability of compounds in marketed amorphous drug products; Eur. J. Pharm. and Biopharm.,?112?204 [Focus Citation]
Properties of Troglitazone
| Melting point: | 184-186°C |
| Boiling point: | 657.0±55.0 °C(Predicted) |
| Density | 1.266±0.06 g/cm3(Predicted) |
| storage temp. | Inert atmosphere,Store in freezer, under -20°C |
| solubility | DMSO: 20 mg/mL |
| form | solid |
| pka | 6.34±0.50(Predicted) |
| color | white to yellow |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20°C for up to 3 months. |
| CAS DataBase Reference | 97322-87-7(CAS DataBase Reference) |
Safety information for Troglitazone
Computed Descriptors for Troglitazone
| InChIKey | GXPHKUHSUJUWKP-UHFFFAOYSA-N |
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
 Troglitazone CAS 97322-87-7View Details
Troglitazone CAS 97322-87-7View Details
97322-87-7 -
 Troglitazone CAS 97322-87-7View Details
Troglitazone CAS 97322-87-7View Details
97322-87-7 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1