Sofosbuvir
- CAS NO.:1190307-88-0
- Empirical Formula: C22H29FN3O9P
- Molecular Weight: 529.45
- MDL number: MFCD18782704
- EINECS: 695-717-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 21:30:31

What is Sofosbuvir?
Absorption
When given orally, sofosbuvir reaches its maximum plasma concentration in about 0.5 to 2 hours with a maximal concentration (Cmax) of 567 ng/mL .
Toxicity
Sofosbuvir, as a single agent, has very mild toxicity. The most common adverse reactions are headache and fatigue. The FDA Label currently warns of a risk of symptomatic bradycardia when Epclusa is used in combination with amiodarone .
Description
Sofosbuvir is a drug used for the treatment of hepatitis C. It is recommended to be used in combination with other drugs (such as velpatasvir) for the first-line treatment for HCV genotypes 1, 2, 3, 4, 5, and 6. It takes effect through acting as a nucleotide analog inhibitor, being capable of specially inhibiting the HCV NS5B (non-structural protein 5B) RNA-dependent RNA polymerase.
Originator
Pharmasset (United States)
The Uses of Sofosbuvir
PSI-7977 is a prodrug that is metabolized to the active antiviral agent 2'-deoxy-2'-α-fluoro-β-C-methyluridine-5'-monophosphate and is currently being investigated in phase 3 clinical trials for the t reatment of hepatitis C. Studies have profiled PSI-7977 as a nucleotide inhibitor of hepatitis C virus, exerting selective inhibitory effects towards HCV NS5B polymerase.
The Uses of Sofosbuvir
PSI-7977 is a phosphoramidate prodrug of PSI-7851, a nucleoside analog that, when phosphorylated, inhibits the RNA-dependent RNA polymerase of hepatitis C virus (EC50 = 92 nM). PSI-7977 is effective in vitro and in vivo.[Cayman Chemical]
The Uses of Sofosbuvir
PSI-7977 is a prodrug that is metabolized to the active antiviral agent 2''-deoxy-2''-α-fluoro-β-C-methyluridine-5''-monophosphate and is currently being investigated in phase 3 clinical trials for the treatment of hepatitis C. Studies have profiled PSI-7977 as a nucleotide inhibitor of hepatitis C virus, exerting selective inhibitory effects towards HCV NS5B polymerase.
Indications
Sofosbuvir is used in combination therapy with other antiviral medications to treat chronic hepatitis C virus (HCV) infected patients with HCV genoptypes 1-6, and to treat HCV and HIV co-infected patients. Depending on the level of cirrhosis or decompensation, combination therapy can also include either ribavirin alone or ribavirin and peg-interferon alfa.
When used in combination with Ledipasvir, sofosbuvir has the following indications: treatment of genotypes 1, 4, 5, or 6 infection without cirrhosis or with compensated cirrhosis; in combination with Ribavirin for genotype 1 infection with decompensated cirrhosis; or in combination with Ribavirin for the treatment of genotype 1 or 4 infection who are liver transplant recipients without cirrhosis or with compensated cirrhosis.
When used in combination with Velpatasvir as the combination product Epclusa, sofosbuvir is indicated for the treatment of adult patients with chronic hepatitis C virus (HCV) genotypes 1, 2, 3, 4, 5, or 6 infection without cirrhosis or with compensated cirrhosis, or in combination with Ribavirin if associated with decompensated cirrhosis.
Resistance: Reduced susceptibility to sofosbuvir has been associated with the NS5B substitution mutation S282T .
Background
Sofosbuvir (tradename Sovaldi) is a direct acting antiviral medication used as part of combination therapy to treat chronic Hepatitis C, an infectious liver disease caused by infection with Hepatitis C Virus (HCV). HCV is a single-stranded RNA virus that is categorized into nine distinct genotypes, with genotype 1 being the most common in the United States, and affecting 72% of all chronic HCV patients . Treatment options for chronic Hepatitis C have advanced significantly since 2011, with the development of Direct Acting Antivirals (DAAs) such as sofosbuvir. As a prodrug nucleotide analog, Sofosbuvir is metabolized into its active form as the antiviral agent 2'-deoxy-2'-α-fluoro-β-C-methyluridine-5'-triphosphate (also known as GS-461203), which acts as a defective substrate for NS5B (non-structural protein 5B) . NS5B, an RNA-dependent RNA polymerase, is essential for the transcription of Hepatitis C viral RNA and for its high replicative rate and genetic diversity . Sofosbuvir and other direct acting antivirals are therefore very potent options for the treatment of Hepatitis C, as they exhibit a high barrier to the development of resistance . This is an important advantage relative to HCV drugs that target other viral enzymes such as the protease, for which rapid development of resistance has proven to be an important cause of therapeutic failure.
In a joint recommendation published in 2016, the American Association for the Study of Liver Diseases (AASLD) and the Infectious Diseases Society of America (IDSA) recommend Sofosbuvir as first line therapy in combination with other antivirals for all six genotypes of Hepatitis C . Depending on the genotype, sofosbuvir is often used in combination with other antivirals such as Ledipasvir, Velpatasvir, Daclatasvir, Simeprevir, Elbasvir, Grazoprevir, Ribavirin, Peginterferon alfa-2a, or Peginterferon alfa-2b with the intent to cure, or achieve a sustained virologic response (SVR), after 12 weeks of daily therapy. SVR and eradication of HCV infection is associated with significant long-term health benefits including reduced liver-related damage, improved quality of life, reduced incidence of Hepatocellular Carcinoma, and reduced all-cause mortality . Treatment with direct acting antivirals such as sofosbuvir is associated with very minimal side effects, with the most common being headache and fatigue . Lack of significant side effects and short duration of therapy is a considerable advantage over older interferon- and ribavirin-based regimens, which were limited by infusion site reactions, reduced blood count, and neuropsychiatric effects .
Since 2014, sofosbuvir has been available as a fixed dose combination product with Ledipasvir (tradename Harvoni) used for the treatment of chronic Hepatitis C. Approved in October 2014 by the FDA, Harvoni is indicated for the treatment of HCV genotypes 1, 4, 5, and 6 with or without Ribavirin depending on the level of liver damage or cirrhosis . When combined together, ledipasvir and sofosbuvir as the combination product Harvoni has been shown to achieve a SVR between 93 and 99% after 12 weeks of treatment . Its use has also proven successful in the treatment of HCV in patients co-infected with HIV .
Sofosbuvir is also available as a fixed dose combination product with Velpatasvir as the commercially available product Epclusa. First approved in June 2016, Epclusa is the first combination HCV product indicated for the treatment of all genotypes of Hepatitis C with or without cirrhosis. Epclusa is also currently the most potent HCV antiviral medication on the market with a sustained virologic response (SVR) after 12 weeks of therapy of 93-99% depending on genotype and level of cirrhosis . Both Canadian and American guidelines list Epclusa as a first line recommendation for all genotypes of HCV .
Notably, sofosbuvir has come under intense scrutiny since its release to market in 2013. With the price per pill set at $1000, a 12-week treatment can cost upwards of $84,000 per patient .
Definition
ChEBI: A nucleotide conjugate that is used in combination with ledipasvir (under the trade name Harvoni) for the treatment of chronic hepatitis C genotype 1 infection.
brand name
Sovaldi
Clinical Use
In December 2013, sofosbuvir (also known as GS-7977 and PSI-7977) was approved in the United States for the treatment of hepatitis C virus (HCV) infection as a component of a combination antiviral treatment regimen. Sofosbuvir was discovered from an effort to enhance the activity of the parent nucleoside by bypassing rate-limiting monophosphorylation with a prodrug that would liberate the intactmonophosphate in the liver, where it would then be converted by cellular kinases to the active triphosphate species. In addition, the prodrug was designed to be amenable to oral delivery. In the initial synthesis of sofosbuvir, the iso-propyl ester of (L)-alanine was coupled with phenyl dichlorophosphate to provide a diastereomeric intermediate that was coupled with the uridine nucleoside. The diastereomeric mixture (GS-9851) was shown to produce high levels of triphosphate in vitro in primary hepatocytes and in the livers of rats, dogs, and monkeys after oral dosing. The individual diastereomers were obtained by chromatography or by crystallization. The diastereomer with the Sp configuration at the phosphorous center (sofosbuvir) was found to be >10-fold more potent in an HCV replicon assay than the corresponding Rp diastereomer (EC90s of 0.42 and 7.5 μM, respectively).
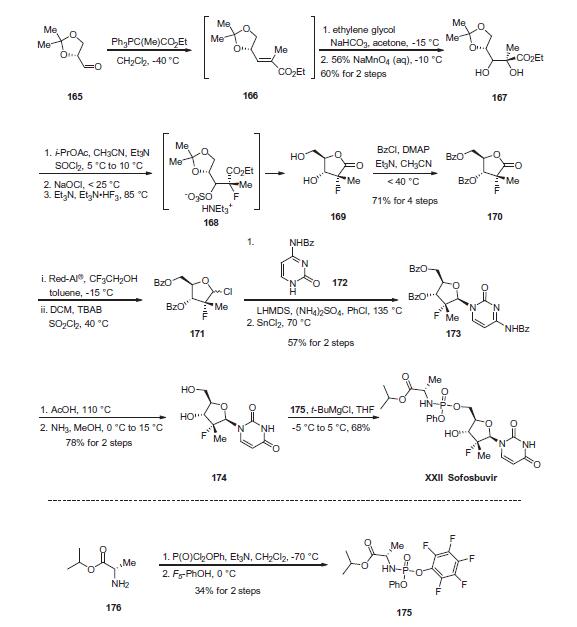
Synthesis
The enantiopure unsaturated ester 166, which readily arises
from olefination of the commercially-available aldehyde 165, was
subjected to ethylene glycol-promoted permanganate dihydroxylation
conditions to afford diol 167 in 60% yield over the two
steps. A three-step sequence was then employed to generate
lactone 169. Diol 167 was converted to the cyclic sulfite and then
oxidized with bleach to give the corresponding cyclic sulfate.
Treatment with nucleophilic fluorine gave intermediate ammonium
sulfonate 168, which, upon acidic hydrolysis of both the acetonide
and sulfonate, underwent cyclization to give lactone 169.
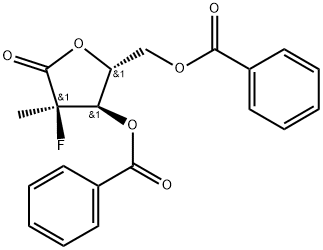
Next, bis-protection of diol 169 furnished 170 in 71% yield for
the four-step sequence. Reduction and chlorination through the
use of Red-Al ® and sulfuryl chloride, respectively, constructed
chlorotetrahydrofuran 171, which was subsequently reacted with
commercial N-(2-oxo-1,2-dihydropyrimidin-4-yl)benzamide 172
in the presence of base and Lewis acid to afford 173 in 57% yield
over the two steps. Treatment of 173 with AcOH and then ammonia
in MeOH removed all benzoyl protection to give rise to diol 174
in 78% yield. Finally, treatment of 174 with pentafluorophenolic
phosphonate ester 175 and tert-butylmagnesium chloride generated
sofosbuvir (XXII) in 68% yield. The final step proceeds with
excellent chirality transfer from 175 (99.7% ee). Notably, the preparation
of the key phosphonate fragment was achieved in a simple
two-step sequence beginning with alanine isopropyl ester 176.
Phosphorylation in the presence of base at cryogenic temperatures,
followed by treatment with pentafluorophenol, delivered scale
quantities of 176 in 34% isolated yield and high enantiopurity
(>98% ee) after recrystallization.
Drug interactions
Potentially hazardous interactions with other drugs
Anti-arrhythmics: possibly increased risk of
bradycardia with amiodarone.
Chemical property
Sofosbuvir [chemical name: 20-Deoxy-20-fluoro-20-Cmethyluridine 50-Sp-[Phenyl(isopropoxy-L-alaninyl)]phosphate] is a low molecular weight (529.46 g/mol), phosphoramidate prodrug with the empirical formula C22H29FN3O9P. Sofosbuvir is a weak acid [acid dissociation constant (pKa) of 9.3], is nonionizable over the physiological pH range, with aqueous solubility C2 mg/mL at 37 ℃, and log Po/w (1-octanol/ water partition coefficient) of 1.6, and as such, is a high solubility and low-permeability Biopharmaceutics Classification System Class 3 compound.
History
Sofosbuvir was discovered in 2007 by Michael Sofia at Pharmasset (now part of Gilead Sciences) and approved for use in the United States by the Food and Drug Administration in 2013. The FDA approval was for the combination of sofosbuvir and ribavirin, an antiviral that dates back to 1972. Sofosbuvir is the active ingredient in Gilead’s products Sovaldi and Harvoni.
Metabolism
In vitro studies in human liver microsomes showed that sofosbuvir was an efficient substrate for Cathepsin A (Cat A) and carboxyl esterase 1 (CES1). Sofosbuvir was cleaved by CatA and CES1 and subsequent activation steps included amino acid removal by histidine triad nucleotide-binding protein 1 (HINT1) and phosphorylation by uridine monophosphate-cytidine monophosphate (UMP-CMP) kinase and nucleoside diphosphate (NDP) kinase. In vitro data indicated that Cat A preferentially hydrolysed sofosbuvir (the S-diastereomer) while CES1 did not exhibit stereoselectivity .
Metabolism
Sofosbuvir is a nucleotide prodrug that is extensively
metabolised. The active metabolite is formed in
hepatocytes and not observed in plasma. The
predominant (>90%) metabolite, GS-331007, is
inactive. It is formed through sequential and parallel
pathways to the formation of active metabolite. The
metabolic pathway involves sequential hydrolysis of the
carboxyl ester moiety catalysed by human cathepsin A
or carboxylesterase 1 and phosphoramidate cleavage
by histidine triad nucleotide binding protein followed
by phosphorylation by the pyrimidine nucleotide
biosynthesis pathway.
Dephosphorylation results in the formation of nucleoside
metabolite GS-331007 that cannot be efficiently
rephosphorylated and lacks anti-HCV activity in vitro.
Following a single 400 mg oral dose of [14C]-sofosbuvir,
mean total recovery of the dose was >92%, consisting of
approximately 80%, 14%, and 2.5% recovered in urine,
faeces, and expired air, respectively. This data indicate
that renal clearance is the major elimination pathway for
GS-331007 with a large part actively secreted.
Properties of Sofosbuvir
| Melting point: | 122-124°C |
| Density | 1.41 |
| storage temp. | Hygroscopic, -20°C Freezer, Under inert atmosphere |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| appearance | white to off-white crystals or powder |
| pka | 9.39±0.10(Predicted) |
| color | White |
| CAS DataBase Reference | 1190307-88-0 |
Safety information for Sofosbuvir
Computed Descriptors for Sofosbuvir
| InChIKey | TTZHDVOVKQGIBA-YBSJRAAASA-N |
| SMILES | C(OC(C)C)(=O)[C@@H](N[P@@](OC[C@@H]1[C@@H](O)[C@](F)(C)[C@H](N2C(=O)NC(=O)C=C2)O1)(OC1=CC=CC=C1)=O)C |
Sofosbuvir manufacturer
SRINI PHARMACEUTICALS PVT LTD
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

![4-[[4-[[4-[(E)-2-cyanoethenyl]-2,6-dimethyl-phenyl]amino]pyrimidin-2-yl]amino]benzonitrile](https://img.chemicalbook.in/CAS/GIF/500287-72-9.gif)
You may like
-
 1190307-88-0 Sofosbuvir Impurity 98%View Details
1190307-88-0 Sofosbuvir Impurity 98%View Details
1190307-88-0 -
 Sofosbuvir 99%View Details
Sofosbuvir 99%View Details -
 Sofosbuvir 99%View Details
Sofosbuvir 99%View Details -
 Sofosbuvir 98%View Details
Sofosbuvir 98%View Details -
 Sofosbuvir 98% (HPLC) CAS 1190307-88-0View Details
Sofosbuvir 98% (HPLC) CAS 1190307-88-0View Details
1190307-88-0 -
 SofosbuvirView Details
SofosbuvirView Details
1190307-88-0 -
 Sofosbuvir Pharma APIView Details
Sofosbuvir Pharma APIView Details
1190307-88-0 -
 Sofosbuvir PowderView Details
Sofosbuvir PowderView Details
1029877-94-8
