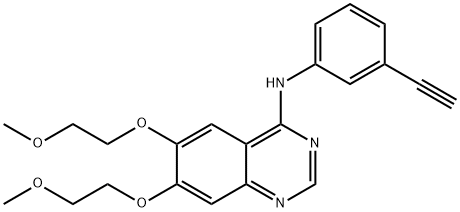
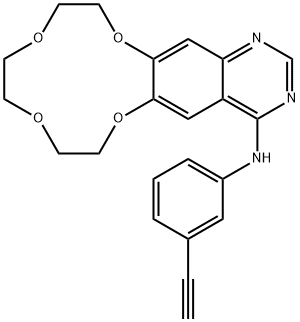
Icotinib
- CAS NO.:610798-31-7
- Empirical Formula: C22H21N3O4
- Molecular Weight: 391.42
- MDL number: MFCD22124501
- EINECS: 1592732-453-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 21:30:31
What is Icotinib?
Description
Icotinib is also known as ConMana. It is a highly selective, first-generation inhibitor of epidermal growth factor receptor tyrosine kinase (EGFR-TKI), whose mutation and overexpression is involved in many kinds of cancer. Icotinib is a quinazoline derivative that binds reversibly to the ATP binding site of the EGFR protein, being capable of blocking the signal transduction cascade. It is currently under investigation for its treatment efficacy on the EGFR+ Non-small cell lung cancer.
The Uses of Icotinib
Icotinib is a potent and specific epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) with IC50 of 5 nM.
Indications
Icotinib (Conmana?, BetaPharma), an EGFR inhibitor, was approved by the China State FDA in 2011 for the treatment of NSCLC. Icotinib resembles erlotinib in possessing the N-(3-ethylnylphenyl) quinazolin-4-amine core scaffold of erlotinib that binds to the EGFR ATP pocket. An adjacent hydrophobic group was also retained while the solvent exposed two 2-methoxyethyoxysubstituents at the 6- and 7-positions of the quinazoline core were cyclized to afford the tetraoxacyclododecene moiety of icotinib. Efficacy and safety of icotinib as first-line therapy in patients has been evaluated for advanced NSCLC in recent clinical studies.
General Description
Class: receptor tyrosine kinase
Treatment: NSCLC
Oral bioavailability = 52%
Elimination half-life = 5.5 h
References
Tan, F., et al. "Icotinib (BPI-2009H), a novel EGFR tyrosine kinase inhibitor, displays potent efficacy in preclinical studies. " Lung Cancer76.2(2012):177-82.
Shi, Y., et al. "Icotinib versus gefitinib in previously treated advanced non-small-cell lung cancer (ICOGEN): a randomised, double-blind phase 3 non-inferiority trial." Lancet Oncology 14.10(2013):953-61.
Sun, Y., Y. Shi, and L. Zhang. "A randomized, double-blind phase III study of icotinib versus gefitinib in patients with advanced non-small cell lung cancer (NSCLC) previously treated with chemotherapy (ICOGEN)."Journal of Thoracic Oncology 29.15(2011):-.
https://en.wikipedia.org/wiki/Icotinib
Properties of Icotinib
| Boiling point: | 581℃ |
| Density | 1.31 |
| Flash point: | 305℃ |
| storage temp. | Store at -20°C |
| solubility | insoluble in H2O; ≥129.6 mg/mL in DMSO; ≥14.13 mg/mL in EtOH with ultrasonic |
| form | Powder |
| pka | 5.32±0.20(Predicted) |
| color | White to off-white |
Safety information for Icotinib
Computed Descriptors for Icotinib
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 Icotinib CAS 610798-31-7View Details
Icotinib CAS 610798-31-7View Details
610798-31-7 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8