Sulfamic acid
Synonym(s):Sulfamic Acid;Aminosulfonic acid;Sulphamic acid;Amidosulfonic Acid;Amidosulfonic acid, Sulphamic acid, Sulfamidic acid
- CAS NO.:5329-14-6
- Empirical Formula: H3NO3S
- Molecular Weight: 97.09
- MDL number: MFCD00011603
- EINECS: 226-218-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
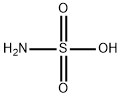
What is Sulfamic acid ?
Chemical properties
Sulfamic acid is a white orthorhombic flaky crystal, odorless, non-volatile and non-hygroscopic. Soluble in water and liquid ammonia, slightly soluble in methanol, insoluble in ethanol and ether, also insoluble in carbon disulfide and liquid sulfur dioxide. Its aqueous solution has the same strong acid properties as hydrochloric acid and sulfuric acid, but its corrosiveness to metals is much lower than that of hydrochloric acid. The toxicity is extremely small, but it should not be in contact with the skin for a long time, and it should not enter the eyes.
The Uses of Sulfamic acid
Sulfamic acid, the monoamide of sulfuric acid, is moderately strong. This compound is used in the synthesis of sweeteners and therapeutic agents. It is generally used in chemical cleaning processes like the removal of nitrites and carbonate- and phosphate-containing deposits.
Sulfamic acid can be used as a catalyst in:
Friedlander quinoline synthesis.
Liquid Beckmann rearrangement for the synthesis of amides from ketoximes.
The preparation of α-aminophosphonates via a three-component reaction between aldehydes, amines, and diethyl phosphite.
Industry, Sulfamic acid is widely used in electroplating, hard-water scale removers, acidic cleaning agents, chlorine stabilizers, sulfonating agents, denitrification agents, disinfectants, flame retardants, herbicides, artificial sweeteners and catalysts.
Sulfamic acid is a precursor to sweet-tasting compounds. Reaction with cyclohexylamine followed by adding NaOH gives C6H11NHSO3Na, sodium cyclamate.
Definition
ChEBI: Sulfamic acid is the simplest of the sulfamic acids consisting of a single sulfur atom covalently bound by single bonds to hydroxy and amino groups and by double bonds to two oxygen atoms. It is a strong acid, readily forming sulphamate salts, which is extremely soluble in water and normally exists as the zwitterion H3N+. SO3–.
Reactions
Sulfamic acid is a strong acid that reacts with many basic compounds. It is heated to above the melting point (209°C) under normal pressure to begin to decompose, and continues to be heated to above 260°C to decompose into sulfur trioxide, sulfur dioxide, nitrogen, hydrogen and water.
(1) Sulfamic acid can react with metals to form transparent crystalline salts. Such as:
2H2NSO3H+Zn→Zn(SO3NH2)2+H2.
(2) Can react with metal oxides, carbonates and hydroxides:
FeO+2HSO3NH2→Fe(SO3NH2)2+H2O2
CaCO3+2HSO3NH2→Ca(SO3NH2)2+H2O+CO23
Ni(OH)2+2HSO3NH2→Ni(SO3NH2)2+H2O.
(3) Can react with nitrate or nitrite:
HNO3+HSO3NH2→H2SO4+N2O+H2O2
HNO2+HSO3NH2→H2SO4+N2+H2O.
(4) Can react with oxidants (such as potassium chlorate, hypochlorous acid, etc.):
KClO3+2HSO3NH2→2H2SO4+KCl+N2+H2O2
2HOCl+HSO3NH2→HSO3NCl2+2H2O
General Description
Sulfamic acid appears as a white crystalline solid. Density 2.1 g / cm3. Melting point 205°C. Combustible. Irritates skin, eyes, and mucous membranes. Low toxicity. Used to make dyes and other chemicals. It is used as a raw material for the preparation of a synthetic sweetener i.e, sodium cyclohexylsulfamate.
Air & Water Reactions
Moderately soluble in water [Hawley].
Reactivity Profile
Sulfamic acid reacts exothermically with bases. Aqueous solutions are acidic and corrosive.
Hazard
Toxic by ingestion.
Health Hazard
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
Flammability and Explosibility
Non flammable
Side Effects
Dermatotoxin - Burning of the skin.
Toxic Pneumonia - Inflammation of the lungs caused by inhalation of metal fumes or toxic gases and vapours.
Safety Profile
Poison by intraperitoneal route. Moderately toxic by ingestion. A human skin irritant. A corrosive irritant to skin, eyes, and mucous membranes. A substance that migrates to food from packaging materials. Violent or explosive reactions with chlorine, metal nitrates + heat, metal nitrites + heat, fuming HNO3. When heated to decomposition it emits very toxic fumes of SOx and NOx. See also SULFONATES.
Toxicology
Sulfamic acid can be absorbed into the body through inhalation of its aerosols and ingestion, causing symptoms of coughing and sore throat upon inhalation; it is irritating to the skin and eyes with symptoms of redness, pain or burns; Inhaling sulfamic acid can irritate the nose and throat. High exposure to sulfamic acid can irritate the lungs. Higher exposures may cause a build-up of fluid in the lungs (pulmonary edema), a medical emergency. sulfamic acid can cause nausea, vomiting and stomach pain. Repeated exposure to sulfamic acid may affect the liver and kidneys.
Potential Exposure
Sulfamic acid is used in metal and ceramic cleaning, bleaching paper pulp; and textiles metal; in acid cleaning; as a stabilizing agent for chlorine and hypochlorite in swimming pools; cooling towers; and paper mills.
Synthesis
A reactor surmounted by a cooler and fitted with an efficient cooling system is charged with 167.7 parts by weight of 100% sulfuric acid and 352 parts of trichlorotrifluoroethane. Then, a stoichiometric quantity of urea, 100.8 parts of industrial grade product, is added progressively at a temperature of 35°C for one and one-half hours. To the suspension of urea sulfate in the fluorocarbon, there is then added, over 45 minutes, 193.9 parts of SO3 as a 42.2% solution in trichlorotrifluoroethane. Thus, a 40% excess of SO3 compared with the stoichiometric amount is added. The temperature rises to 47°-48°C., the boiling temperature of the fluorocarbon. The complex formed between the three reagents, a liquid insoluble in the trichlorotrifluoroethane, is separated by decanting. By heating to 85°C., this complex is decomposed until all the CO2 and the excess SO3 have been given off, and 314.6 parts of a product are obtained. This product contains 96.2% sulfamic acid, corresponding to a production of crude sulfamic acid in a 93% yield.
Shipping
UN2967 Sulfamic acid, Hazard class: 8; Labels: 8-Corrosive material.
Purification Methods
Crystallise NH2SO3H from water at 70o (300mL per 25g), after filtering, by cooling a little and discarding the first batch of crystals (about 2.5g) before standing in an ice-salt mixture for 20minutes. The crystals are filtered off by suction, washed with a small quantity of ice cold water, then twice with cold EtOH and finally with Et2O. Dry it in air for 1hour, then store it in a desiccator over Mg(ClO4)2 [Butler et al. Ind Eng Chem (Anal Ed) 10 690 1938]. For the preparation of primary standard material see Pure Appl Chem 25 459 1969.
Incompatibilities
The aqueous solution is a strong acid. Reacts violently with strong acids (especially fuming nitric acid), bases, chlorine. Reacts slowly with water, forming ammonium bisulfate. Incompatible with ammonia, amines, isocyanates, alkylene oxides; epichlorohydrin, oxidizers.
Properties of Sulfamic acid
| Melting point: | 215-225 °C (dec.) (lit.) |
| Boiling point: | -520.47°C (estimate) |
| Density | 2.151 g/cm3 at 25 °C |
| vapor pressure | 0.8Pa at 20℃ |
| refractive index | 1.553 |
| storage temp. | Store below +30°C. |
| solubility | water: soluble213g/L at 20°C |
| form | Crystals or Crystalline Powder |
| pka | -8.53±0.27(Predicted) |
| color | White |
| PH | 1.2 (10g/l, H2O) |
| Odor | odorless |
| Water Solubility | 146.8 g/L (20 ºC) |
| Merck | 14,8921 |
| Stability: | Stable. |
| CAS DataBase Reference | 5329-14-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Sulfamic acid(5329-14-6) |
| EPA Substance Registry System | Sulfamic acid (5329-14-6) |
Safety information for Sulfamic acid
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Sulfamic acid
| InChIKey | IIACRCGMVDHOTQ-UHFFFAOYSA-N |
Sulfamic acid manufacturer
Amrutha Organics
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran




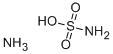
You may like
-
 SULPHAMIC ACID 98%View Details
SULPHAMIC ACID 98%View Details -
 Sulfamic acid 98%View Details
Sulfamic acid 98%View Details -
 Amidosulfonic acid CAS 5329-14-6View Details
Amidosulfonic acid CAS 5329-14-6View Details
5329-14-6 -
 Amidosulfonic acid CAS 5329-14-6View Details
Amidosulfonic acid CAS 5329-14-6View Details
5329-14-6 -
 Sulphamic Acid extrapure AR CAS 5329-14-6View Details
Sulphamic Acid extrapure AR CAS 5329-14-6View Details
5329-14-6 -
 Sulphamic Acid PowderView Details
Sulphamic Acid PowderView Details
5329-14-6 -
 5329-14-6 Sulfamic Acid Powder, For Descaling Agent, 99%View Details
5329-14-6 Sulfamic Acid Powder, For Descaling Agent, 99%View Details
5329-14-6 -
 Powder Sulfamic Acid For Dye and Pigment Manufacturing, For IndustrialView Details
Powder Sulfamic Acid For Dye and Pigment Manufacturing, For IndustrialView Details
5329-14-6
