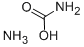
Ammonium sulfamate
Synonym(s):Amidosulfonic acid ammonium salt, Ammonium sulfamate, Sulfamic acid ammonium salt;Ammonium amidosulfonate
- CAS NO.:7773-06-0
- Empirical Formula: H6N2O3S
- Molecular Weight: 114.12
- MDL number: MFCD00011429
- EINECS: 231-871-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
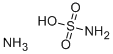
What is Ammonium sulfamate?
Description
Ammonium sulfamate (or ammonium sulphamate) is a white crystalline solid, readily soluble in water. It is a salt formed from ammonia and sulfamic acid. Based on its good performance, it is widely used in industrial production and scientific research. Ammonium sulfamate is used as a herbicide, a composting accelerator in horticultural settings, a flame retardant, and so on.
Chemical properties
Ammonium sulfamate is a white to yellow crystalline solid.
The Uses of Ammonium sulfamate
Ammonium sulfamate (AMS) is an inorganic herbicide used for control of woody plants and herbaceous perennials.
What are the applications of Application
Ammonium sulfamate could be widely used in pesticides, printing and dyeing, tobacco, building materials, textiles, and other industries. It could used as an analytical reagent. It could also used in the manufacture of fire-retardant compositions, for flameproofing textiles and paper products, and in the manufacture of weed-killing compositions. It is also used in electroplating solutions and could used for the generation of nitrous oxide gas.
What are the applications of Application
Ammonium Sulfamate is a weakly oxidizing agent employed as a broad-spectrum herbicide
Definition
ChEBI: Ammonium sulfamate is an organic molecular entity.
Preparation
Ammonium sulfamate is prepared continuously, starting from ammonia and sulfur trioxide, in which liquid sulfur trioxide and liquid ammonia are introduced in a molar ratio NH3 /SO3 greater than 2.0:1 into a pressure reactor containing the reaction product in the molten state, excess ammonia being removed continuously from the reactor and the liquid sulfur trioxide being introduced into the melt.
General Description
Ammonium sulfamate is a white crystalline solid. Ammonium sulfamate is soluble in water. The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment. Ammonium sulfamate is used to flameproof fabrics and papers, in weed or brush killing products, and for other uses.
Air & Water Reactions
Water soluble. Hot water [Note: Elevated temperatures cause a highly exothermic reaction with water (in the presence of acid)] .
Reactivity Profile
Ammonium sulfamate is incompatible with the following: Acids, hot water [Note: Elevated temperatures cause a highly exothermic reaction with water (in the presence of acid).] .
Hazard
Hot acid solutions when enclosed may explode.
Health Hazard
Inhalation causes irritation of nose and throat. Ingestion causes gastrointestinal disturbances. Dust irritates eyes.
Fire Hazard
Special Hazards of Combustion Products: Toxic oxides of nitrogen may form in fires.
Safety Profile
Moderately toxic by ingestion and intraperitoneal routes. Somewhat explosive when heated or by spontaneous chemical reaction in a hot acid solution. A powerful oxidizer. When heated to decomposition it emits very toxic fumes of NH3, NOx, and SOx. See also SULFONATES and SULFAMIC ACID.
Potential Exposure
Ammonium sulfamate is used as a herbicide and in compositions for retarding flame in textiles and paper products; a softener for paper, cotton textiles.
Carcinogenicity
The oral LD50 values were 3900 mg/kg for rats and 5760 mg/kg for mice.
Shipping
UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.
Purification Methods
Crystallise it from water at room temperature (1mL/g) by adding EtOH and cooling. [Sisler & Audrieth Inorg Synth II 180 1946.]
Incompatibilities
Strong oxidizers, potassium, potassium chlorate, sodium nitrite, metal chlorates, and hot acid solutions. Elevated temperatures cause a highly exothermic reaction with water.
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. Dilute with water, make neutral with acid or base and flush into sewer with more water.
Properties of Ammonium sulfamate
| Melting point: | 131-135 °C (lit.) |
| Boiling point: | 160°C |
| Density | 1.769 |
| vapor pressure | 0Pa at 20℃ |
| Flash point: | 160°C |
| storage temp. | no restrictions. |
| solubility | H2O: 1 M at 20 °C, clear, colorless |
| form | Crystalline Powder |
| color | White |
| Odor | Slight amine odor |
| PH | 5.0-6.0 (50g/l, H2O, 20℃) |
| PH Range | 4.5 - 6.0 |
| Water Solubility | 1950 g/L (20 ºC) |
| Sensitive | Air & Moisture Sensitive |
| Merck | 14,554 |
| Exposure limits | ACGIH: TWA 10 mg/m3 OSHA: TWA 15 mg/m3; TWA 5 mg/m3 NIOSH: IDLH 1500 mg/m3; TWA 10 mg/m3; TWA 5 mg/m3 |
| Stability: | Stable. Incompatible with strong acids, strong oxidizing agents. |
| CAS DataBase Reference | 7773-06-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Ammonium sulfamate(7773-06-0) |
| EPA Substance Registry System | Ammonium sulfamate (7773-06-0) |
Safety information for Ammonium sulfamate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H336:Specific target organ toxicity,single exposure; Narcotic effects H400:Hazardous to the aquatic environment, acute hazard |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P271:Use only outdoors or in a well-ventilated area. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P330:Rinse mouth. P405:Store locked up. P403+P233:Store in a well-ventilated place. Keep container tightly closed. P501:Dispose of contents/container to..… |
Computed Descriptors for Ammonium sulfamate
| InChIKey | GEHMBYLTCISYNY-UHFFFAOYSA-N |
Ammonium sulfamate manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 Ammonium sulfamate, For ACS analysis CAS 7773-06-0View Details
Ammonium sulfamate, For ACS analysis CAS 7773-06-0View Details
7773-06-0 -
 Ammonium sulfamate, For ACS analysis CAS 7773-06-0View Details
Ammonium sulfamate, For ACS analysis CAS 7773-06-0View Details
7773-06-0 -
 Ammonium sulphamate, GR 99%+ CAS 7773-06-0View Details
Ammonium sulphamate, GR 99%+ CAS 7773-06-0View Details
7773-06-0 -
 Ammonium Amidosulfate CAS 7773-06-0View Details
Ammonium Amidosulfate CAS 7773-06-0View Details
7773-06-0 -
 Ammonium Sulphamate (High Purity) extrapure AR CAS 7773-06-0View Details
Ammonium Sulphamate (High Purity) extrapure AR CAS 7773-06-0View Details
7773-06-0 -
 Ammonium sulphamate CAS 7773-06-0View Details
Ammonium sulphamate CAS 7773-06-0View Details
7773-06-0 -
 Ammonium Sulphamate (High Purity) ACS CAS 7773-06-0View Details
Ammonium Sulphamate (High Purity) ACS CAS 7773-06-0View Details
7773-06-0 -
 AMS 32 Inch Infrared (IR) Touch Screen MonitorView Details
AMS 32 Inch Infrared (IR) Touch Screen MonitorView Details
7773-06-0
