Ethyl 2-(Chlorosulfonyl)acetate
- CAS NO.:55896-93-0
- Empirical Formula: C4H7ClO4S
- Molecular Weight: 186.61
- MDL number: MFCD08458866
- EINECS: 818-924-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-09 10:27:52

What is Ethyl 2-(Chlorosulfonyl)acetate?
The Uses of Ethyl 2-(Chlorosulfonyl)acetate
Pharmaceutic aid (acidifying agent).
The Uses of Ethyl 2-(Chlorosulfonyl)acetate
Acetic Acid is an acid produced chemically from the conversion of alcohol to acetaldehyde to acetic acid. it is the principal component of vinegar which contains not less than 4 g of acetic acid in 100 cm3 at 20°c. the approved salts include sodium acetate, calcium acetate, sodium diacetate, and calcium diacetate. it is used as a preservative, acidulant, and flavoring agent in catsup, mayonnaise, and pickles. it can be used in conjunction with leavening agents to release carbon dioxide from sodium bicarbonate.
The Uses of Ethyl 2-(Chlorosulfonyl)acetate
Colorless liquid prepared by the distillation of wood or the
oxidation of dilute ethyl alcohol. Acetic acid has a very pungent
smell, and the vapors are flammable. When obtained in full
strength (99 percent), it congeals as an ice-like solid at 16.7°C.
For this reason the term glacial is also used to describe this acid.
It is soluble in water, alcohol, ether, chloroform, and gelatin.
Acetic acid was used as a restrainer for the physical development
of calotypes, Niépceotypes, and collodion plates. Photographers
also used it to retard non-image reduction of these
processes by adding it to the silver nitrate solution. Acetic acid
is used as a stop bath and as a solvent for gelatin.
brand name
Vosol (Carter-Wallace).
Biotechnological Production
Acetic acid is produced for beverage, food, and feed applications almost entirely
using the traditional vinegar process . First, ethanol is produced by fermentation
with Saccharomyces cerevisiae in the absence of oxygen. Then, acetic acid
is generated from ethanol by acetic acid bacteria, such as Acetobacter aceti,
Acetobacter pasteurianus, or Gluconacetobacter europaeus, under aerobic conditions. Different substrates, such as malt, fruits, and sugarcane, are used
for vinegar production . Today, processes with two stages (e.g. two-tank cycle
fermentation or two-stage submerged fermentation) are generally employed on an
industrial scale. In a first step, biomass is produced in parallel to the acetic acid
production. In the second part of the process, mainly acidification takes place.
Acetic acid concentrations up to 200 g.L-1 can be achieved .
The vinegar process has been well studied over many decades . However,
there are still attempts to enhance vinegar production, especially regarding productivity and cost minimization through alternative substrates, new
process concepts (e.g. immobilized cells or mixed cultures of yeasts and
acetic acid bacteria, and optimized acetic acid bacteria.
Acetic acid can be produced under anaerobic conditions by some microorganisms
such as Clostridium thermoaceticum . In free-cell batch fermentations,
acetate concentrations of 50 g.L-1 were reached in less than 192 h. Acetic
acid concentrations of 83–100 g.L-1, a yield of 0.74–0.80 g acetic acid per gram
glucose, and a productivity of 0.60–0.85 g.L-1.h-1 were observed under optimized
conditions in a cell-recovered fed-batch process with pH-control using
glucose as substrate.
Health Hazard
Recommended Personal Protective Equipment: Protective clothing should be worn when skin contact can occur. Respiratory protection is necessary when exposed to vapor. Complete eye protection is recommended; Symptoms Following Exposure: Breathing of vapors causes coughing, chest pains, and irritation of the nose and throat; may cause nausea and vomiting. Contact with skin and eyes causes burns; General Treatment for Exposure: INHALATION: Move the victim immediately to fresh air. If breathing becomes difficult, give oxygen and get medical attention immediately. INGESTION: If the victim is conscious, have him drink water or milk. Do not induce vomiting. SKIN OR EYE CONTACT: Flush immediately with lots of clean running water; wash eyes for at least 15 min. and get medical attention as quickly as possible; remove contaminated clothing; Toxicity by Inhalation (Threshold Limit Value): 10 ppm; Short-Term Exposure Limits: 40 ppm for 5 min.; Toxicity by Ingestion: LD50 0.5 to 5.0 g/kg (rat); Late Toxicity: No data; Vapor (Gas) Irritant Characteristics: Vapors cause moderate irritation such that workers will find high concentrations very unpleasant. Effects are temporary; Liquid or Solid Irritant Characteristics: This is a fairly severe skin irritant; may cause pain and secondary burns after a few minutes of contact; Odor Threshold: 1.0 ppm.
Chemical Reactivity
Reactivity with Water No reaction; Reactivity with Common Materials: Corrosive, particularly when diluted. Attacks most common metals including most stainless steels. Excellent solvent for many synthetic resins or rubber; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Dilute with water, rinse with sodium bicarbonate solution; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.
Properties of Ethyl 2-(Chlorosulfonyl)acetate
| Melting point: | 16.2 °C(lit.) |
| Boiling point: | 117-118 °C(lit.) |
| Density | 1.049 g/mL at 25 °C(lit.) |
| vapor density | 2.07 (vs air) |
| vapor pressure | 11.4 mm Hg ( 20 °C) |
| refractive index | n |
| Flash point: | 104 °F |
| storage temp. | Inert atmosphere,2-8°C |
| solubility | Benzene, Chloroform |
| form | Liquid |
| pka | 4.76(at 25℃) |
| color | Colorless |
| Odor | Characteristic vinegar, pungent; vinegar-like; sharp. |
| Relative polarity | 0.648 |
| Exposure limits | ACGIH: TWA 10 ppm; STEL 15 ppm OSHA: TWA 10 ppm(25 mg/m3) NIOSH: IDLH 50 ppm; TWA 10 ppm(25 mg/m3); STEL 15 ppm(37 mg/m3) |
| Stability: | Moisture Sensitive, Hygroscopic |
| CAS DataBase Reference | 55896-93-0(CAS DataBase Reference) |
Safety information for Ethyl 2-(Chlorosulfonyl)acetate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for Ethyl 2-(Chlorosulfonyl)acetate
Ethyl 2-(Chlorosulfonyl)acetate manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


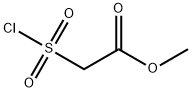
![(1,3,7-TRIMETHYL-2,4-DIOXO-1,2,3,4-TETRAHYDRO-IMIDAZO[2,1-F]PURIN-8-YL)-ACETIC ACID](https://img.chemicalbook.in/StructureFile/ChemBookStructure8/GIF/CB6336242.gif)
![([1-(2-PHENOXYETHYL)-1H-BENZIMIDAZOL-2-YL]THIO)ACETIC ACID](https://img.chemicalbook.in/StructureFile/ChemBookStructure3/GIF/CB5333714.gif)
You may like
-
 Chlorosulfonyl acetic acid ethyl ester 97% CAS 55896-93-0View Details
Chlorosulfonyl acetic acid ethyl ester 97% CAS 55896-93-0View Details
55896-93-0 -
 Acetic Acid Food GradeView Details
Acetic Acid Food GradeView Details
55896-93-0 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
