Folic acid
Synonym(s):Folic acid;Cyanocobalamin;Vitamin M;Pteroylglutamic acid;Pterylmonoglutamic acid
- CAS NO.:59-30-3
- Empirical Formula: C19H19N7O6
- Molecular Weight: 441.4
- MDL number: MFCD00079305
- EINECS: 200-419-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 21:30:31

What is Folic acid?
Absorption
Folic acid is absorbed rapidly from the small intestine, primarily from the proximal portion. Naturally occurring conjugated folates are reduced enzymatically to folic acid in the gastrointestinal tract prior to absorption. Folic acid appears in the plasma approximately 15 to 30 minutes after an oral dose; peak levels are generally reached within 1 hour.
Toxicity
IPR-MUS LD50 85 mg/kg,IVN-GPG LD50 120 mg/kg, IVN-MUS LD50 239 mg/kg, IVN-RAT LD50 500 mg/kg, IVN-RBT LD50 410 mg/kg
Description
Folic acid, also known as folate or Vitamin B9, is a member of the B vitamin family and an essential cofactor for enzymes involved in DNA and RNA synthesis. More specifically, folic acid is required by the body for the synthesis of purines, pyrimidines, and methionine before incorporation into DNA or protein. It is essential for many bodily functions, including DNA synthesis, repair, and methylation. Folic acid is particularly important during phases of rapid cell division, such as infancy, pregnancy, and erythropoiesis, and plays a protective factor in the development of cancer. As humans are unable to synthesize folic acid endogenously, diet and supplementation is necessary to prevent deficiencies. For example, folic acid is present in green vegetables, beans, avocado, and some fruits.
Chemical properties
orange to yellow crystalline powder
Physical properties
Folates comprise a large group of chemically related species differing in substituents at three sites of the pteroylglutamic acid structure, with over 170 theoretically possible forms and an estimated 100 occurring in animals. Natural folates typically carry a single carbon unit at N-5 and/or N-10, participating in one-carbon metabolism. Folic acid (pteroylmonoglutamic acid) is an orange-yellow crystalline compound soluble in water but insoluble in ethanol or less polar organic solvents. It is unstable to light, acidic/alkaline conditions, reducing agents, and heat (except in dry form). It is enzymatically reduced in vivo (or chemically with reductants like dithionite) first to 7,8-dihydrofolic acid (FH?) and then to FH?, both requiring antioxidants (e.g., ascorbic acid) for stability in aerobic environments. Two derivatives with an amino group replacing the C-4 hydroxyl—aminopterin (4-aminofolic acid) and methotrexate (4-amino-N10-methylfolic acid)—serve as folate antagonists in biomedicine, used as a rodenticide and antineoplastic agent, respectively.
Originator
Folvite, Lederle, US ,1946
Occurrence
Synthetic
Indications
Inadequate folate levels can result in several health concerns, including cardiovascular disease, megaloblastic anemias, cognitive deficiencies, and neural tube defects (NTDs). Folic acid is typically supplemented during pregnancy to prevent the development of NTDs and in individuals with alcoholism to prevent the development of neurological disorders, for example. Folic acid is indicated for treating folic acid deficiency, megaloblastic anemia, and anemias of nutritional origins during pregnancy, infancy, or childhood.
What are the applications of Application
Folic Acid is an important cofactor in the transfer of one-carbon moieties plays role in formation of S-adenosyl met
Definition
ChEBI: An N-acyl-amino acid that is a form of the water-soluble vitamin B9. Its biologically active forms (tetrahydrofolate and others) are essential for nucleotide biosynthesis and homocysteine remethylation.
Manufacturing Process
The following description is taken from US Patent 2,956,057.
100 grams of 1,3,3-trichloroacetone are heated on a boiling water bath and
95 grams of bromine are added thereto in drops while being stirred and the
stirring is continued for about 1 hour. The resulting reaction solution is
distilled under reduced pressure. 115 grams of 1-bromo-1,3,3-
trichloroacetone are obtained having a boiling point of 85° to 95°C/17 mm
(Hg).
For the preparation of the hydrate, 100 grams of water are added to 100
grams of 1bromo-1,3,3-trichloroacetone, which is agitated and cooled. A white
scaly crystal of hydrate of 1-bromo-1,3,3-trichloroacetone is obtained (100
grams), having a melting point of 52° to 53°C.
8.9 grams of 2,4,5-triamino-6-hydroxypyrimidine hydrochloride and 8 grams
of p-aminobenzoylglutamic acid are dissolved in 400 cc warm water, which is
cooled at 35° to 27°C and adjusted to pH 4 by using 20% caustic soda
solution. To this solution was simultaneously added dropwise a solution
obtained by dissolving 13.4 grams of 1-bromo-1,3,3-trichloroacetone hydrate
in 90 cc of 50% methanol and 24 grams of 35% aqueous sodium bisulfite
solution over a period of approximately 2 hours. During this period, in order
to maintain the pH value of the reaction solution at 4 to 5, 20% caustic soda
solution is added from time to time. The precipitate, formed by stirring for 5
hours after dropping was finished, is filtered, and the filtrated precipitate is
refined; 5.6 grams of pure pteroylglutamic acid is obtained.
Therapeutic Function
Treatment of B vitamin (folacin) deficiency
General Description
Odorless orange-yellow needles or platelets. Darkens and chars from approximately 482°F.
Air & Water Reactions
Insoluble in water. Aqueous solutions have pHs of 4.0-4.8.
Reactivity Profile
Acid solutions of Folic acid are sensitive to heat, but towards neutrality, stability progressively increases. Solutions are inactivated by ultraviolet light and alkaline solutions are sensitive to oxidation. Folic acid is also inactivated by light. Folic acid is incompatible with oxidizing agents, reducing agents and heavy metal ions.
Fire Hazard
Flash point data for Folic acid are not available; however, Folic acid is probably combustible.
Biochem/physiol Actions
A nutritional delivery form of folate. Folic acid and its derivatives are essential mediators of one-carbon metabolism within cells.
Pharmacokinetics
Folic acid (pteroylglutamic acid; FA) occurs largely as polyglutamates in food and must be hydrolyzed to the monoglutamate form for absorption, a reaction mediated by folate conjugase on the brush border of enterocytes in the proximal small intestine. Absorption of the mono glutamate form occurs in part by a saturable transport process with an acidic pH optimum via the proton-coupled folate transporter (PCFT, SLC46A1) that is expressed widely, including on the brush border membrane of the proximal intestine and renal tubule. PCFT is active only at pH 5–6, as in the microenvironment of those membranes. The Km for this system is 1–3 μM. In addition, an apparently unsaturable pathway is active when luminal concentrations of folate exceed 5–10 μM. This dual system is responsible for part of the variation in bioavailability at the extreme levels of folate intake. The bioavailability of FA was quite low after a high oral dose (F = 0.01), in contrast to a high value after intramuscular administration (F = 0.95). The plasma levels of biologically active, reduced forms of folates (THF and 5MF) were significantly increased over their basal levels after IV and IM administration to FA. The levels of these active folates did not increase after oral administration of a similar dose of FA. A 50 times higher dose was required to increase the active folates to the levels observed after IV and IM administration.
Clinical Use
Folate-deficient megaloblastic anaemia
Supplement in HD patients
Safety Profile
Poison by intraperitoneal and intravenous routes. Experimental teratogenic effects. Mutation data reported. When heated to decomposition it emits toxic fumes of NOx.
Veterinary Drugs and Treatments
Folic acid is used to treat folic acid deficiency in dogs, cats, and horses (theoretically in other animal species as well) often due to small intestinal disease. Cats with exocrine pancreatic insufficiency appear to be most at risk for folate and cobalamin deficiencies secondary to malabsorption of folic acid in the diet. Dogs with exocrine pancreatic insufficiency often are noted to have increased folate levels secondary to overgrowths of folate-synthesizing bacteria in the proximal small intestine. Chronic administration of dihydrofolate reductase inhibiting drugs such as pyrimethamine, ormetoprim or trimethoprim can potentially lead to reduced activated folic acid (tetrahydrofolic acid); folic acid supplementation is sometimes prescribed in an attempt to alleviate this situation.
Drug interactions
Potentially hazardous interactions with other drugs
Antiepileptics: reduces phenytoin, primidone and
phenobarbital levels.
Cytotoxics: avoid with raltitrexed.
Metabolism
Folic acid is metabolized in the liver into the cofactors dihydrofolate (DHF) and tetrahydrofolate (THF) by the enzyme dihydrofolate reductase (DHFR).
Properties of Folic acid
| Melting point: | 250 °C |
| Boiling point: | 552.35°C (rough estimate) |
| alpha | 20 º (c=1, 0.1N NaOH) |
| Density | 1.4704 (rough estimate) |
| refractive index | 1.6800 (estimate) |
| storage temp. | 2-8°C |
| solubility | boiling water: soluble1% |
| form | Crystalline Powder |
| pka | pKa 2.5 (Uncertain) |
| color | Yellow to orange |
| Odor | Odorless |
| PH Range | 4 |
| Water Solubility | 1.6 mg/L (25 ºC) |
| Merck | 14,4221 |
| BRN | 100781 |
| Stability: | Stable. Incompatible with heavy metal ions, strong oxidizing agents, strong reducing agents. Solutions may be light and heat sensitive. |
| CAS DataBase Reference | 59-30-3(CAS DataBase Reference) |
| NIST Chemistry Reference | Folic acid(59-30-3) |
| EPA Substance Registry System | L-Glutamic acid, N-[4-[[(2-amino-3,4-dihydro-4-oxo-6-pteridinyl)methyl]amino]benzoyl]- (59-30-3) |
Safety information for Folic acid
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Folic acid
| InChIKey | OVBPIULPVIDEAO-LBPRGKRZSA-N |
Folic acid manufacturer
Gangwal Healthcare Pvt Ltd
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
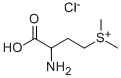
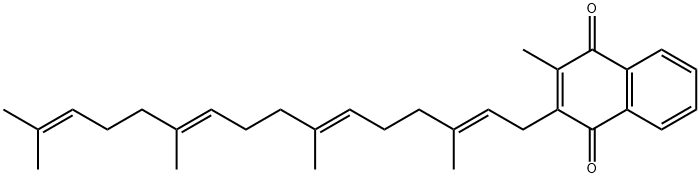
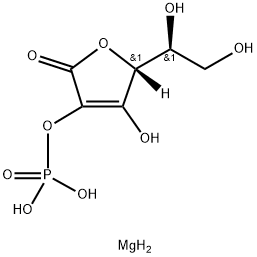
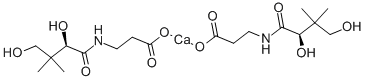
You may like
-
 Folic Acid 99%View Details
Folic Acid 99%View Details -
 Folic acid hydrate 95% CAS 59-30-3View Details
Folic acid hydrate 95% CAS 59-30-3View Details
59-30-3 -
 Folic Acid for cell culture CAS 59-30-3View Details
Folic Acid for cell culture CAS 59-30-3View Details
59-30-3 -
 Folic Acid pure CAS 59-30-3View Details
Folic Acid pure CAS 59-30-3View Details
59-30-3 -
 Vitamin B9 Folic Acid PowderView Details
Vitamin B9 Folic Acid PowderView Details
59-30-3 -
 Folic Acid Cas No. 59 30 3View Details
Folic Acid Cas No. 59 30 3View Details
59-30-3 -
 Folic AcidView Details
Folic AcidView Details
59-30-3 -
 Folic Acid Powder, 25Kg BagView Details
Folic Acid Powder, 25Kg BagView Details
59-30-3
