Zofenopril calcium
Synonym(s):(4S)-1-[(2S)-3-(benzoylthio)-2-methyl-1-oxopropyl]-4-(phenylthio)-L-proline calcium salt
- CAS NO.:81938-43-4
- Empirical Formula: C22H25CaNO4S2
- Molecular Weight: 471.64
- MDL number: MFCD08704648
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
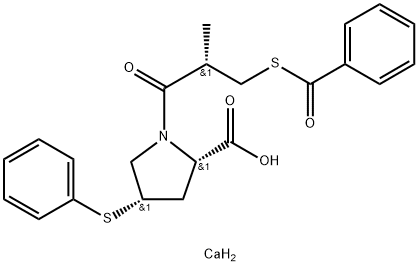
What is Zofenopril calcium?
Description
Zofenopril calcium was introduced as a second-generation angiotensinconverting enzyme (ACE) inhibitor for the treatment of acute myocardial infarction. Zofenopril, rapidly hydrolyzed by cardiac esterase, is actually a S-benzoyl prodrug of the active component zofenopril-sulfhydryl (zofenoprilat), the latter being the ACE inhibitor responsible for the improvement of postischemic contractile function and for the reduction of cardiac cell death. It was suggested that ACE inhibition alone was not sufficient to explain the cardioprotective effects; the antioxidant properties demonstrated in vitro and in viva could partly explain the strong anti-ischemic effects. In rats with CHF after myocardial infarction, zofenopril attenuated ventricular enlargement and cardiac hypertrophy. It was also shown in isolated globally ischemic rat hearts that the cardioprotective effect of zofenopril was stereoselective. Clinical studies in healthy volunteers comparing zofenopril with enalapril demonstrated that the rate of hydrolysis of the former was faster; at 30 or 60 mg, ACE was completely inhibited in most volunteers until 12h after administration. In patients with anterior acute myocardial infarction, a g-week treatment with zofenopril significantly reduced the incidence of death or severe CHF and also improved the chance of surviving the next year.
Description
Zofenopril is a prodrug form of the angiotensin-converting enzyme (ACE) inhibitor zofenoprilat. Zofenopril is hydrolyzed by cardiac esterases in vivo to form zofenoprilat. It inhibits ACE with an IC50 value of 0.9 nM in heart tissue homogenates and inhibits cardiac ACE activity in isolated perfused rat hearts. It reduces mean arterial blood pressure in two kidney-one clip renal hypertensive (2K-1C) rats and spontaneously hypertensive rats (SHRs) when administered at doses of 2.2, 6.6, and 22 mg/kg. Unlike the ACE inhibitor ramipril , zofenopril does not affect bronchoalveolar lavage fluid (BALF) levels of bradykinin or prostaglandin E2 (PGE2; ) or increase coughing induced by citric acid in guinea pigs.
Chemical properties
Off-White to Light Yellow Crystalline Solid
Originator
Bristol-Myers Squibb (US)
The Uses of Zofenopril calcium
Angiotensin-converting enzyme ACE inhibitor. A prodrug that is de-esterified to the active inhibitor, the sulfhydryl group containing metabolite, Zofenoprilat
The Uses of Zofenopril calcium
adenosine A2a receptor agonist
What are the applications of Application
Zofenopril Calcium Salt is an antioxidant that acts as an angiotensin-converting enzyme inhibitor
Definition
ChEBI: An organic calcium salt that is the hemicalcium salt of zofenopril. A prodrug for zofenoprilat.
Manufacturing Process
9.9 g (0.031 mole) of cis-4-phenylthio-L-proline is suspended in 100 ml of
water (pH 5.6) and the pH is adjusted to 10.2 by the addition of about 20 ml
of 10% sodium bicarbonate to provide a clear solution. The pH is then
adjusted to 9.5 by the addition of about 4.5 ml of concentrated HCl. The
solution is kept at 30°C while 8.1 g (0.033 mole) of (D)-3-(benzoylthio)-2-
methylpropanoic acid chloride in 30 ml of toluene is added simultaneously
with 100 ml of 10% sodium bicarbonate to keep the pH at 9.3. After about
1/4 of the acid chloride is added, a slimy precipitate begins to form which
persists throughout the reaction. After stirring the reaction mixture at pH 9.3
for 2.5 h, it is made strongly acidic by adding 20% HCl in the presence of
ethyl acetate. The aqueous layer is extracted twice with 350 ml portions of
ethyl acetate and the combined organic layers are washed with 300 ml of
saturated brine and dried (MgSO 4 ). The solvent is removed to yield 11.8 g of
foamy solid cis-1-[D-3-(benzoylthyo)-2-methyl-1-oxopropyl]-4-(phenylthio)-L-proline hydrochloride.
To a solution of this cis-1-[D-3-(benzoylthyo)-2-methyl-1-oxopropyl]-4-
(phenylthio)-L-proline hydrochloride 11.8 g (0.027 mole) in 70 ml of
acetonitrile there is added about 6.0 g of dicyclohexylamine in 25 ml of ether.
A white crystalline precipitate forms immediately. After standing overnight in
the cold room, the solid is filtered and washed with ether to yield (cis)-1-[D-
3-(benzoylthio)-2-methyl-1-oxopropyl]-4-(phenylthio)-L-proline,
dicyclohexylamine salt (1:1).
he slightly moist (cis)-1-[D-3-(benzoylthio)-2-methyl-1-oxopropyl]-4-
(phenylthio)-L-proline dicyclohexylamine salt is stirred for 2.5 h in a mixture
of 300 ml of ethyl acetate and 200 ml of 10% potassium bisulfate. Two clear
layers form. The aqueous layer is extracted with two 200 ml portions of ethyl
acetate and the combined organic layers are dried (MgSO 4 ). The solvent is
removed to yield 10.1 g of foamy solid (cis)-1-[D-3-(benzoylthio)-2-methyl-1-
oxopropyl]-4-(phenylthio)-L-proline; melting point 42-44°C.
In practice it is usually used as calcium salt (2:1).
brand name
Zoprace (Bristol-Myers Squibb);Zantipres.
Therapeutic Function
Antihypertensive
Properties of Zofenopril calcium
| Melting point: | >250°C |
| alpha | D23 -67.6° (c = 1 in methanol/HCl) |
| storage temp. | Inert atmosphere,2-8°C |
| solubility | DMSO: >5mg/mL |
| form | powder |
| color | white to off-white |
| CAS DataBase Reference | 81938-43-4(CAS DataBase Reference) |
Safety information for Zofenopril calcium
| Signal word | Warning |
| Pictogram(s) |
 Environment GHS09 |
| GHS Hazard Statements |
H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P501:Dispose of contents/container to..… |
Computed Descriptors for Zofenopril calcium
Zofenopril calcium manufacturer
Biophore India Pharmaceuticals Pvt Ltd
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 81938-43-4 Zofenopril calcium 98%View Details
81938-43-4 Zofenopril calcium 98%View Details
81938-43-4 -
 81938-43-4 98%View Details
81938-43-4 98%View Details
81938-43-4 -
 Zofenopril Calcium CAS 81938-43-4View Details
Zofenopril Calcium CAS 81938-43-4View Details
81938-43-4 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1