Zofenoprilat
- CAS NO.:75176-37-3
- Empirical Formula: C15H19NO3S2
- Molecular Weight: 325.45
- MDL number: MFCD00865919
- SAFETY DATA SHEET (SDS)
- Update Date: 2022-12-21 16:56:50
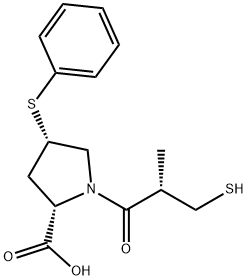
What is Zofenoprilat?
Chemical properties
White crystalline
Originator
Zofenil arginine,Menarini
The Uses of Zofenoprilat
The active metabolite of Zofenopril, an ACE inhibitor
Definition
ChEBI: A proline derivative that is 4-(phenylsulfanyl)-L-proline in which the amine proton is replaced by a (2S)-2-methyl-3-sulfanylpropanoyl group. The active metabolite of zofenopril.
Manufacturing Process
Sodium metal (0.85 g, 0.037 mole) is dissolved in 40 ml of absolute ethanol.
To this there is added with stirring 3.7 ml (0.036 mole) of thiophenol followed
by 7.5 g (0.017 mole) of N-carbobenzyloxy-trans-4-tosyloxy-L-proline, methyl
ester [J. Am. Chem. Soc., 79, 191 (1957)]. After stirring for 4 h and standing
overnight at room temperature, the bulk of the ethanol is removed on a rotary
evaporator. The mostly solid residue is stirred with 120 ml of dichloromethane
and 60 ml of water. The layers are separated (some methanol is added to help
break up emulsions) and the aqueous phase is extracted with additional
dichloromethane (2x60 ml). The combined organic phase are washed with 100
ml of saturated sodium chloride solution, dried (MgSO 4 ), and the solvent
evaporated to give 6.5 g (100%) of N-carbobenzyloxy-cis-4-phenylthio-L-
proline, methyl ester as a pale yellow viscous oil.
The N-carbobenzyloxy-cis-4-phenylthio-L-proline, methyl ester (6.5 g, 0.017
mole) is dissolved in 55 ml of methanol, treated portionwise at -1° to 4°C
with 13 ml (0.026 mole) of 2 N sodium hydroxide, stirred at 0°C for 1 h, and
kept at room temperature for approximately 16 h. After removing about half
of the solvent on a rotary evaporator, the cooled solution is diluted with 100
ml of water, washed with 60 ml of ether (wash discarded), layered over with
70 ml of ethyl acetate, stirred, cooled, and acidified with 4.8 ml of 1:1
hydrochloric acid. After separating, the aqueous phase is extracted with
additional ethyl acetate (3x40 ml) and the combined organic layers are dried
(MgSO 4 ) and evaporated to give 5.9 g of a light yellow viscous oil. The latter
is dissolved in 30 ml of ethanol, treated with 1.9 g of cyclohexylamine in 3 ml
of ethanol and diluted to 330 ml with ether. On seeding, the crystalline
cyclohexylamine salt separates. The latter, after cooling for approximately 16
h, weighs 5.3 g; melting point 148-151°C. This material is combined with 1.5
g of identical product from a previous experiment, stirred with 200 ml of
boiling acetonitrile, and cooled to yield 6.3 g of colorless N-carbobenzyloxy-
cis-4-phenylthio-L-proline cyclohexylamine salt; melting point 152-155°C.
This N-carbobenzyloxy-cis-4-phenylthio-L-proline cyclohexylamine salt is
suspended in 25 ml of ethyl acetate, stirred, and treated with 25 ml of 1 N
hydrochloric acid. When two clear layers are obtained, they are separated and
the aqueous phase is extracted with additional ethyl acetate (3x25 ml). The
combined organic layers are dried (MgSO 4 ) and the solvent evaporated to give 5.0 g (65%) of N-carbobenzyloxy-cis-4-phenylthio-L-proline as a nearly
colorless, very viscous syrup.
N-Carbobenzyloxy-cis-4-phenylthio-L-proline (4.9 g, 0.014 mole) is treated
with 25 ml of hydrogen bromide in acetic acid (30-32%), stoppered loosely,
and stirred magnetically. After 1 h the orange-yellow solution is diluted to 250
ml with ether to precipitate the product as a heavy oil which gradually
crystallizes on seeding, rubbing and cooling After stirring in an ice-bath for 1
h, the material is filtered under nitrogen, washed with ether, suspended in
fresh ether, cooled for approximately 16 h, and filtered again to give 3.2 g
(77%) of colorless solid (cis)-4-phenylthio-L-proline hydrobromide; melting
point 106-109°C.
A solution of 3.0 g (0.0094 mole) of (cis)-4-phenylthio-L-proline hydrobromide
in 25 ml of water is stirred, cooled to 5°C and 15 ml of 20% sodium
carbonate are added. This mixture is treated with 2.0 g (0.011 mole) of D-3-
acetylthio-2-methylpropionyl chloride in 5 ml of ether during the course of 10
min with the intermittent addition of 3.0 g of sodium carbonate to maintain
the pH at 8.0 to 8.4). The mixture is stirred in the ice-bath for an additional
hour, 25 ml of water are added and then a solution of 5 ml of concentrated
hydrochloric acid in 25 ml of water. The strongly acid solution is saturated
with sodium chloride and extracted with 50 ml of ethyl acetate (four times).
The organic phases are combined, dried, filtered and solvent evaporated to
give 3.8 g of a pale yellow viscous oil. The dicyclohexylamine salt following
trituration with 15 ml of acetonitrile one obtains 2.4 g of colorless solid 1-[D-
3-(acetylthio)-2-methyl-1-oxopropyl]-cis-4-phenylthio-L-proline
dicyclohexylamine salt; melting point 184-186°C.
Therapeutic Function
Antihypertensive
Properties of Zofenoprilat
| Boiling point: | 556.2±50.0 °C(Predicted) |
| Density | 1.31±0.1 g/cm3(Predicted) |
| pka | 3.32±0.40(Predicted) |
Safety information for Zofenoprilat
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H311:Acute toxicity,dermal H331:Acute toxicity,inhalation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P310:Immediately call a POISON CENTER or doctor/physician. P330:Rinse mouth. P361:Remove/Take off immediately all contaminated clothing. P302+P352:IF ON SKIN: wash with plenty of soap and water. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P405:Store locked up. P403+P233:Store in a well-ventilated place. Keep container tightly closed. P501:Dispose of contents/container to..… |
Computed Descriptors for Zofenoprilat
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8