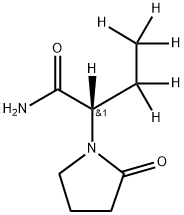
Levetiracetam
Synonym(s):(αS)-α-Ethyl-2-oxo-1-pyrrolidineacetamide;2(S)-(2-Oxopyrrolidin-1-yl)butyramide;Levetiracetam
- CAS NO.:102767-28-2
- Empirical Formula: C8H14N2O2
- Molecular Weight: 170.20896
- MDL number: MFCD03265610
- EINECS: 600-348-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 18:10:20
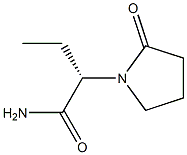
What is Levetiracetam?
Absorption
Levetiracetam is rapidly and nearly completely absorbed following oral administration, with a reported absolute oral bioavailability of essentially 100%. Tmax is approximately 1.3 hours after dosing, and Cmax is 31 μg/mL following a single 1000mg dose and 43 μg/mL following repeated dosing. Co-administration of levetiracetam with food delays Tmax by approximately 1.5 hours and decreases Cmax by 20%.
Toxicity
The oral TDLO of levetiracetam in humans is 10 mg/kg. Symptoms of levetiracetam overdose are consistent with its adverse effect profile and may include agitation, aggression, somnolence, decreased level of consciousness, respiratory depression, or coma. There is no antidote for levetiracetam overdose, therefore management should involve general supportive measures and symptomatic treatment. Hemodialysis results in significant clearance of plasma levetiracetam (approximately 50% within 4 hours) and should be considered in cases of overdose as indicated by the patient's status.
Description
Levetiracetam was first introduced in the US as an adjunctive therapy in the treatment of partial-onset seizures in adults with epilepsy. This second-generation analog of piracetam can be prepared by condensation of (S)-2-aminobutyramide with 4-chlorobutyryl chloride. Although its mechanism of action is not well established, it was shown that [3H]-levetiracetam reversibly binds to a specific site predominantly present in the membranes of the brain. Unlike conventional anticonvulsants such as phenytoin, carbamazepine, valproic acid, phenobarbital, diazepam and clonazepam, compounds structurally-related to levetiracetam, such as piracetam and aniracetam, also have affinity for this site. Levetiracetam reveals a broad and unique profile in animal seizure models, including promising antiepileptogenic properties. Besides being rapidly and almost completely absorbed in man (oral bioavailability>95%), it possesses a favorable pharmacokinetic profile since it is not hepatically metabolized but only partly hydrolized into the inactive carboxylic acid by enzymes in a number of tissues including blood cells, it is minimally bound to plasma proteins (<10%) and does not inhibit or induce hepatic enzymes. Therefore levetiracetam has a low potential for drug interaction, providing a useful alternative as adjunctive therapy to treat seizures refractory to conventional anticonvulsants.
Chemical properties
White Crystalline Solid
Originator
UCB (Belgium)
The Uses of Levetiracetam
The (S)-enantiomer of the ethyl analog of Piracetam. Used as an anticonvulsant.
The Uses of Levetiracetam
The (S)-enantiomer of Etiracetam (E932970) and the ethyl analog of Piracetam (P500800). Used as an anticonvulsant.
The Uses of Levetiracetam
A compound which inhibits burst firing without affecting normal neuronal excitability
The Uses of Levetiracetam
Used as adjunctive therapy in the treatment of partial onset seizures in adults and children 4 years of age and older with epilepsy.
Background
Levetiracetam is a drug within the pyrrolidine class that is used to treat various types of seizures stemming from epileptic disorders. It was first approved for use in the United States in 1999 and is structurally and mechanistically unrelated to other anti-epileptic drugs (AEDs). Levetiracetam possesses a wide therapeutic index and little-to-no potential to produce, or be subject to, pharmacokinetic interactions - these characteristics make it a desirable choice over other AEDs, a class of drugs notorious for having generally narrow therapeutic indexes and a propensity for involvement in drug interactions.
Indications
Levetiracetam is indicated as an adjunctive therapy in the treatment of partial onset seizures in epileptic patients who are one month of age and older. Additionally, it is indicated as an adjunct in the treatment of myoclonic seizures in patients with juvenile myoclonic epilepsy who are 12 years of age and older, and in primary generalized tonic-clonic seizures in patients with idiopathic generalized epilepsy who are 6 years of age and older.
Levetiracetam is also available as an orally dissolvable tablet that is indicated as an adjunct in the treatment of partial onset seizures in patients with epilepsy who are 4 years of age and older and weigh more than 20kg.
What are the applications of Application
Levetiracetam is a compound which inhibits burst firing without affecting normal neuronal excitability
Definition
ChEBI: A pyrrolidinone and carboxamide that is N-methylpyrrolidin-2-one in which one of the methyl hydrogens is replaced by an aminocarbonyl group, while another is replaced by an ethyl group (the S enantiomer). An anticonvulsa t, it is used for the treatment of epilepsy in both human and veterinary medicine.
Manufacturing Process
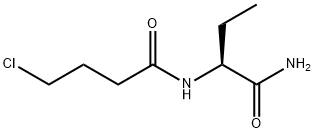
(a) Preparation of the (R)-α-methyl-benzylamine salt of (S)-α-ethyl-2-oxo-1-
pyrrolidineacetic acid
8.7 kg (50.8 moles) of racemic ()-α-ethyl-2-oxo-1-pyrrolidineacetic acid are
suspended in 21.5 liters of anhydrous benzene in a 50 liter reactor. To this
suspension is added gradually a solution containing 3.08 kg (25.45 moles) of
(R)-(+)-α-methyl-benzylamine and 2.575 kg (25.49 moles) of triethylamine in
2.4 liters of anhydrous benzene. This mixture is then heated to reflux
temperature until complete dissolution. It is then cooled and allowed to
crystallize for a few hours. 5.73 kg of the (R)-α-methyl-benzylamine salt of
(S)-α-ethyl-2-oxo-1-pyrrolidineacetic acid are thus obtained. Melting point:
148°-151°C. Yield: 77.1%.
This salt may be purified by heating under reflux in 48.3 liters of benzene for
4 hours. The mixture is cooled and filtered to obtain 5.040 kg of the desired
salt. Melting point: 152°-153.5°C. Yield: 67.85%.
(b) Preparation of (S)-α-ethyl-2-oxo-1-pyrrolidineacetic acid
5.04 kg of the salt obtained in (a) above are dissolved in 9 liters of water. 710
g of a 30% sodium hydroxide solution are added slowly so that the pH of the
solution reaches 12.6 and the temperature does not exceed 25°C. The
solution is stirred for a further 20 minutes and the α-methylbenzylamine
liberated is extracted with a total volume of 18 liters of benzene. The aqueous
phase is then acidified to a pH of 1.1 by adding 3.2 liters of 6 N hydrochloric
acid. The precipitate formed is filtered off, washed with water and dried. The
filtrate is extracted repeatedly with a total volume of 50 liters of
dichloromethane. The organic phase is dried over sodium sulfate and filtered
and evaporated to dryness under reduced pressure. The residue obtained after
the evaporation and the precipitate isolated previously, are dissolved together
in 14 liters of hot dichloromethane. The dichloromethane is distilled and
replaced at the distillation rate, by 14 liters of toluene from which the product
crystallizes. The mixture is cooled to ambient temperature and the crystals
are filtered off to obtain 2.78 kg of (S)-α-ethyl-2-oxo-1-pyrrolidineacetic acid.
Melting point: 125.9°C. [α]D20 = -26.4° (c = 1, acetone). Yield: 94.5%.
(c) Preparation of (S)-α-ethyl-2-oxo-1-pyrrolidineacetamide
34.2 g (0.2 mole) of (S)-α-ethyl-2-oxo-1-pyrrolidineacetic acid are suspended
in 225 ml of dichloromethane cooled to -30°C. 24.3 g (0.24 mole) of
triethylamine are added dropwise over 15 minutes. The reaction mixture is
then cooled to -40°C and 24.3 g (0.224 mole) of ethyl chloroformate are
added over 12 minutes. Thereafter, a stream of ammonia is passed through
the mixture for 4 ? hours. The reaction mixture is then allowed to return to
ambient temperature and the ammonium salts formed are removed by
filtration and washed with dichloromethane. The solvent is distilled off under
reduced pressure. The solid residue thus obtained is dispersed in 55 ml
toluene and the dispersion is stirred for 30 minutes and then filtered. The
product is recrystallized from 280 ml of ethyl acetate in the presence of 9 g of
0.4 nm molecular sieve in powder form 24.6 g of (S)-α-ethyl-2-oxo-1-
pyrrolidineacetamide are obtained. Melting point: 115°-118°C. [α]D25 = -89.7°
(c = 1, acetone). Yield: 72.3%.
brand name
Keppra (UCB).
Therapeutic Function
Antiepileptic, Nootropic
Biological Functions
Levetiracetam (Keppra) has recently been approved for the treatment of partial-onset seizures. It appears to be safe and effective; its exact therapeutic profile has yet to be determined. It does not appear to share any of the mechanisms of action of agents that have been discussed to this point. It does have a highly specific brain binding site, but the significance of this observation to its mechanism of action has not been elucidated.
General Description
LEV is an analog of the nootropic agent, piracetam. Onlythe S-isomer has any anticonvulsant activity. Unlike piracetam, LEV does not have any affinity for the AMPA receptor thereby has no nootropic activity forthe treatment of Alzheimer disease. LEV also has no affinityfor GABA receptors, BZD receptors, the various excitatoryamino acid related receptors, or the voltage-gated ionchannels.For this reason, its mechanism of anticonvulsantaction remains unclear, but it appears to exert itsantiepileptic action by modulating kainite/AMPA-inducedexcitatory synaptic currents, thus decreasing membraneconductance.Furthermore, the anticonvulsant activity ofthis drug appears to be mediated by the parent moleculerather than by its inactive metabolite,(S)-α-ethyl-2-oxo-1-pyrrolidineacetic acid (i.e., via the hydrolysis of amidegroup).Like gabapentin, LEV has few drug interactionswith other AEDs thereby can be used in combination to treatrefractory epilepsy.
Biological Activity
Antiepileptic that displays distinctive properties from conventional antiepileptic drugs. Displays potent seizure protection in animal models of chronic epilepsy but lacks activity in acute seizure models. Binds synaptic vesicle protein 2A (SV2A) and inhibits Na + -dependent Cl - /HCO 3 - exchange.
Biochem/physiol Actions
Levetiracetam is a pyrrolidine with antiepileptic activity. Stereoselective binding of levetiracetam was confined to synaptic plasma membranes in the central nervous system with no binding occurring in peripheral tissue. Levetiracetam inhibits burst firing without affecting normal neuronal excitability, which suggests that it may selectively prevent hyper-synchronization of epileptiform burst firing and propagation of seizure activity.
Mechanism of action
The mechanism of action for S-(–)-levetiracetam is unknown. It does not appear to interact with any of the recognized excitatory or inhibitory neural mechanism. A CNS-specific binding site for S-(–)-levetiracetam has been identified as the synaptic vesicle protein (SV2A). Knockout animals without SV2A proteins accumulated presynaptic Ca2+ during consecutive action potentials that destabilized synaptic circuits and induced epilepsy. Thus, it appears that SV2A plays a major role in the antiepileptic properties of S-(–)-levetiracetam, which acts by modulating the function of SV2A and the regulation of Ca2+ mediated synaptic transmission. These data support previous indications that S-(–)-levetiracetam possesses a mechanism of action distinct from that of other antiepileptic drugs. Three SV2 isoforms (SV2A, SV2B, and SV2C) have been identified, each of which has a unique distribution in brain, suggesting synapse-specific functions as well as antagonism of neuronal synchronization.
Pharmacokinetics
Levetiracetam is rapidly and almost completely absorbed after oral administration. Levetiracetam tablets and oral solutions are bioequivalent. The pharmacokinetics are linear and time-invariant, with low intra- and inter-subject variability. The extent of bioavailability of levetiracetam is not affected by food. Levetiracetam is not significantly protein-bound (<10% bound), and its volume of distribution is close to the volume of intracellular and extracellular water. Sixty-six percent (66%) of the dose is renally excreted unchanged. The major metabolic pathway of levetiracetam (24% of dose) is an enzymatic hydrolysis of the acetamide group. It is not liver cytochrome P450 dependent. The metabolites have no known pharmacological activity and are renally excreted. The plasma half-life of levetiracetam across studies is approximately 6-8 hours. It is increased in the elderly (primarily due to impaired renal clearance) and in subjects with renal impairment.
Pharmacokinetics
S-(–)-levetiracetam displays rapid and complete absorption, although food slows the rate but not the extent of absorption. It exhibits linear pharmacokinetics and is minimally protein bound. Approximately 60% of an oral dose is excreted into the urine unchanged and 24 to 30% as its carboxylic acid metabolite, with an elimination half-life in adults of approximately 7 hours. Although S-(–)-levetiracetam is not metabolized by hepatic CYP450, UGT, or epoxide hydrolase, it is esterase hydrolyzed to its carboxylic acid metabolite (loss of amido group), which is not affected by the hepatic metabolizing enzymes.
Clinical Use
S-(–)-levetiracetam is a pyrrolidone derivative unrelated to the structures of other AEDs. It is indicated as an adjunct in the treatment of partial onset seizures in adults, and it has shown some benefit in clinical trials for generalized tonic-clonic seizures (GTC) and myoclonic seizures in adults and children.
Side Effects
The risk of clinically relevant drug interactions is minimal with S-(–)-levetiracetam, because it does not alter the pharmacokinetics of coadministered drugs by inhibition or induction of hepatic enzymes. Toxic effects include mild to moderate somnolence, asthenia, ataxia, and dizziness; these effects seldom require discontinuance. An increase in the incidence of behavioral abnormalities in children and in adults having a previous history of neuropsychiatric problems has been noted. Its use in the elderly or in patients with renal impairment will require an individualization of dose, and an additional dose is needed after renal dialysis. Levetiracetam was associated with developmental toxicity in the offspring of pregnant animals.
Veterinary Drugs and Treatments
Levetiracetam may be useful as a third antiseizure medication in
dogs that are not well controlled with phenobarbital and bromides
or when either bromides or phenobarbital are not tolerated. Some
evidence suggests that in dogs suffering from phenobarbital liver
toxicity, the addition of levetiracetam will allow reduction of their
phenobarbital dosage without increasing seizure frequency.
Levetiracetam may also be useful as add-on therapy in cats.
Side Effects
The most common side effects of levetiracetam (LEV) are neurobehavioral, including fatigue, nervousness, generalized weakness, irritability, agitation, emotional lability, depression, mood swings, vertigo, anxiety, unsteadiness, seizures, memory loss, confusion, increased reflexes, paresthesias, aggression, cognitive decline, and increased risk of suicide. Other common side effects include hypersensitivity reactions, infections, myalgias, rhinitis, and anorexia. Neurobehavioral side effects are the main cause of discontinuing the medications in most instances. There are several case reports and case series that report the acute onset of psychosis with the initiation of LEV. Increased risk of suicide has also been reported in patients on LEV therapy.
Metabolism
Levetiracetam is minimally metabolized within the body - the major metabolic pathway appears to be the enzymatic hydrolysis of its acetamide group which produces an inactive carboxylic acid metabolite, L057, which accounts for approximately 24% of the total administered dose. The specific enzyme(s) responsible for this reaction are unclear, but this pathway is known to be independent of hepatic CYP enzymes and has been proposed to be driven primarily by type B esterases in the blood and other tissues. Two minor metabolites involving modifications to the pyrrolidone ring have been identified, one involving hydroxylation of the ring (constituting 1.6% of the total dose) and the other involving opening of the ring structure (constituting 0.9% of the total dose).
Storage
Store at RT
References
1) Wright?et al.?(2013)?Clinical Pharmacology and Pharmacokinetics of Levetiracetam; Front. Neurol.?4?192 2) De Smedt?et al.?(2007)?Levetiracetam: the profile of a novel anticonvulsant drug – part I: preclinical data; CNS Drug Rev.?13?43 3) Klitgaard and Verdru (2007)?Levetiracetam: the first SV2A ligand for the treatment of epilepsy; Drug Discov.?21537 4) Lynch?et al.?(2004)?The synaptic vesicle protein SV2A is the binding site for the antiepileptic drug levetiracetam; Proc. Natl. Acad. Sci. USA?101?9861 5) Nagarkatti et al. (2008)?Levetiracetam inhibits both ryanodine and IP3 receptor activated calcium induced calcium release in hippocampal neurons in culture; Neurosci. Lett.?436?289 6) Bonnet?et al.?(2019)?Levetiracetam mediates subtle pH-shifts in adult human pyramidal cells via an inhibition of the bicarbonate-driven neuronal pH-regulation – Implications for excitability and plasticity modulation; Brain Res.?1710?146
Properties of Levetiracetam
| Melting point: | 118-119°C |
| alpha | -89.7 º |
| Flash point: | 9℃ |
| storage temp. | 2-8°C |
| solubility | H2O: >5mg/mL |
| form | powder |
| color | white |
| optical activity | [α]/D -90±5°, c = 1 in acetone |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20° for up to 1 month. |
| InChI | InChI=1/C8H14N2O2/c1-2-6(8(9)12)10-5-3-4-7(10)11/h6H,2-5H2,1H3,(H2,9,12)/t6-/s3 |
| CAS DataBase Reference | 102767-28-2(CAS DataBase Reference) |
Safety information for Levetiracetam
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Levetiracetam
| InChIKey | HPHUVLMMVZITSG-UHFFFAOYSA-N |
| SMILES | C(N)(=O)[C@@H](N1CCCC1=O)CC |&1:3,r| |
Levetiracetam manufacturer
SRINI PHARMACEUTICALS PVT LTD
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 Levetiracetam 99%View Details
Levetiracetam 99%View Details -
 Levetiracetam 98%View Details
Levetiracetam 98%View Details -
 Levetiracetam 98%View Details
Levetiracetam 98%View Details -
 Levetiracetam 98%View Details
Levetiracetam 98%View Details -
 Levetiracetam 98%View Details
Levetiracetam 98%View Details -
 Levetiracetam 98%View Details
Levetiracetam 98%View Details -
 Levetiracetam Api Powder, 99%View Details
Levetiracetam Api Powder, 99%View Details
102767-28-2 -
 Levetiracetam Api Powder, 99%View Details
Levetiracetam Api Powder, 99%View Details
102767-28-2
