Zolmitriptan
Synonym(s):(4S)-4-((3-(2-(Dimethylamino)ethyl)-1H-indol-5-yl)methyl)-2-oxazolidinone;(S)-4-((3-(2-(Dimethylamino)ethyl)indol-5-yl)methyl)-2-oxazolidinone;311C90
- CAS NO.:139264-17-8
- Empirical Formula: C16H21N3O2
- Molecular Weight: 287.36
- MDL number: MFCD00871503
- EINECS: 629-919-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-11 08:41:34
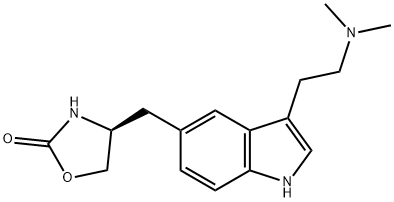
What is Zolmitriptan?
Absorption
Zolmitriptan tablets have a mean absolute oral bioavailability of approximately 40%, with food having no effect on the rate or extent of absorption. The dosing kinetics are linear over a range of 2.5 to 50 mg with 75% of the eventual Cmax being attained within 1 hour of dosing. The median Tmax for the tablet form is 1.5 hours, while for the orally disintegrating tablet form, it is 3 hours. The AUC across studies was in the range of 84.4-173.8 ng/mL*h while the Cmax was between 16 and 25.2 ng/mL.
Zolmitriptan administered as a nasal spray is detected in the plasma within 2-5 minutes, compared to 10-15 minutes for the tablet form; the faster kinetics likely reflect fast absorption across the nasal mucosa. The bioavailability compared to the tablet is 102%, and plasma zolmitriptan concentration is maintained for 4-6 hours after intranasal delivery.
The active N-desmethyl metabolite of zolmitriptan has a mean plasma concentration that is roughly two-thirds of zolmitriptan, regardless of dosage route or concentration.
Toxicity
Toxicity information regarding zolmitriptan is not readily available. Patients experiencing an overdose are at an increased risk of severe adverse effects such as cardiovascular symptoms due to excessive vasoconstriction and activation of serotonergic receptors. Patients receiving a single 50 mg oral dose of zolmitriptan often experienced sedation. Symptomatic and supportive measures are recommended.
Description
Zolmitriptan is a selective serotonin receptor agonist of the 1B and 1D subtypes. It is mainly used in the acute treatment of migraine attacks with or without aura and cluster headaches. Zolmitriptan takes effect through binding to human 5-HT1Band 5-HT1Dreceptors, leading to cranial blood vessel constriction and the release of sensory neuropeptides through nerve endings in the trigeminal system.
Chemical properties
White Crystalline Powder
Originator
Zeneca (UK)
The Uses of Zolmitriptan
Zolmitriptan is a serotonin 5HTID-receptor agonist and used to treat migraine (1,2,3).
The Uses of Zolmitriptan
adrenergic agonist, nasal decongestant
What are the applications of Application
Zolmitriptan is a serotonin 5HTID-receptor agonist
Background
Zolmitriptan is a member of the triptan class of 5-hydroxytryptamine(5-HT)1B/1D/(1F) receptor agonists used to treat acute migraine. Sumatriptan was the first triptan to be developed, but had poor oral bioavailability and lipophilicity. This led to the development of second-generation triptans, including almotriptan, eletriptan, frovatriptan, naratriptan, rizatriptan, and zolmitriptan. Triptans can be administered alone or in combination with an NSAID like naproxen, and represent the current "gold standard" for acute migraine treatment.
Zolmitriptan was first approved by the FDA for sale by Zeneca Pharmaceuticals under the trade name Zomig? on November 25, 1997. It is currently available in both tablet and nasal spray forms.
Indications
Zolmitriptan is indicated for the acute treatment of migraine with or without auras in patients aged 18 and over.
Definition
ChEBI: Zolmitriptan is a member of the class of tryptamines that is N,N-dimethyltryptamine in which the hydrogen at position 5 of the indole ring has been replaced by a [(4S)-2-oxo-1,3-oxazolidin-4-yl]methyl group. A serotonin 5-HT1 B and D receptor agonist, it is used for the treatment of migraine. It has a role as a serotonergic agonist, a vasoconstrictor agent and an anti-inflammatory drug. It is a member of tryptamines and an oxazolidinone. It is functionally related to a N,N-dimethyltryptamine.
Manufacturing Process
(S)-4-(4-[N'-(2-Oxotetrahydropyran-3-iliden)hidrazino]benzyl}-1,3-oxazolidin-
2-one
A solution of 2.8 g (40.6 mmoles) of sodium nitrite in 12 ml of water was
added slowly to a solution of 9.1 g (39.8 mmoles) of (S)-4-(4-aminobenzyl)-
1,3-oxazolidyne-2-one hydrochloride in 17 ml of water and 29 ml of
concentrated HCl, keeping the reaction temperature below 0°C. The mixture was stirred at this temperature for 15 minutes. Once that time had elapsed
the diazonium salt solution was added rapidly to a suspension of 30 g (239
mmoles) of sodium sulphite in 106 ml of water precooled to 0°C under
nitrogen atmosphere. The red solution was stirred at 0°C for 10 minutes and
then left to reach 65°C in 1 hour. It was stirred at 65°C for 30 minutes, and
18.2 ml of concentrated HCl then added. The mixture was stirred at the same
temperature under nitrogen atmosphere for 3 hours and then left to cool to
room temperature. To this solution was added a solution of 35 mmoles of α-
keto-γ-valerolactone (prepared by decarboxylation of 11.8 g (63.7 mmoles) of
a ethoxyalyl-γ-butyrolactone in 15.2 ml of 2 N H 2 SO 4 at reflux) and left under
stirring at room temperature for 12 hours. When that time had elapsed the
mixture was cooled to 0°C and stirred for one hour. The precipitate formed
was filtered, washed with cold water and dried in an hotair oven at 40°C,
giving a white solid which was crystallised from ethanol/water to give 10.5 g
(87%) of the title hydrazone as a white solid. Melting point 223.3°-224.7°C.
(S)-6-(2-Oxo-1,3-oxazolidin-4-ylmethyl)-4,9-dihydro-3H-pyrano-[3,4-b]indol-
1-one
3.8 g (12.5 mmoles) of (S)-4-{4-[N'-(2-oxotetrahydropyran-3-iliden)
hydrazino]benzyl}-1,3-oxazolidin-2-one were suspended in 32 ml of a
saturated solution of hydrogen chloride in acetic acid. The mixture was stirred
at room temperature for 16 h, 10 ml of water/ice was added to the reaction
mixture and stirred at 0°C for 20 min. The precipitate was filtered, washed
with cold water and dried in hot-air oven at 40°C. The residue was crystallised
with methanol to yield 3.3 g (92%) of the title indole as a yellow crystalline
solid. Melting point 215°-217°C.
(S)-3-(2-Hydroxyethyl)-5-(2-oxo-1,3-oxazolidin-4-ylmethyl)-1-H-indol-2-
carboxylicacid methyl ester
To a suspension of 500 mg (1.74 mmoles) of the (S)-6-(2-oxo-1,3-oxazolidin-
4-ylmethyl)-4,9-dihydro-3-pyrano-[3,4-b]indol-1-one in 10 ml of methanol
were added 0.12 ml (1.9 mmoles) of methanesulfonic acid. The mixture was
left under stirring at the reflux temperature for 3 hours. The solvent was
evaporated to dryness under reduced pressure, the residue dissolved with 10
ml of a saturated bicarbonate solution and extracted three times with
dichloromethane. The combined organic phases were dried and evaporated to
dryness and the evaporated solid recrystallised from ethanol to give 517 mg
(93%) of the title ester as a yellow crystalline solid. Melting point 178°-
180°C.
(S)-3-(2-Hydroxyethyl)-5-(2-oxo-1,3-oxazolidin-4-ylmethyl)-1H-indol-2-
carboxylicacid ethyl ester
9.5 g (31.3 mmoles) of (S)-4-{4-[N'-(2-oxotetrahydropyran-3-ilyden)
hydrazine]benzyl}-1,3-oxazolidin-2-one were suspended in 76 ml of a 2 N
solution of hydrogen chloride in absolute ethanol. The mixture was left under
stirring at 75°C for 30 min. The solvent was evaporated to dryness under
reduced pressure, 50 ml of a saturated solution of potassium carbonate
added, and then extracted three times with 50 ml of dichloromethane. The
combined organic phases were dried on anhydrous sodium sulphate and recrystallised from methanol to give a yellow crystalline solid. Melting point
154°-156°C.
evaporated to dryness. The residue was recrystallised from isopropyl
alcohol/heptane to give 9.25 g (89%) of the title indole. The product was
brand name
Zomig (AstraZeneca); Zomig (IPR).
Therapeutic Function
Serotoninergic
General Description
Zolmitriptan, the second triptan marketed (approved in1997), has a much better bioavailability (40%–48%) thansumatriptan. It is rapidly absorbed after oral or nasal sprayadministration. It also has an orally disintegrating tablet formulation(Zomig ZMT), which can be taken without water.Zolmitriptan undergoes rapid N-demethylation via CYP1A2to a more potent, active metabolite, N-desmethylzolmitriptan,which is 2 to 6 times more potent than the parentdrug. This active metabolite was detected 5 minutesafter dosing and accounts for about two thirds of the plasmaconcentration of the administered dose of the parent drug.284Thus, it is reasonable to assume that the therapeutic effectsand especially the CNS side effects of zolmitriptan must bein part attributed to the plasma levels of this active metabolite,at least until it is further degraded by hepatic MAO-Ato its inactive indole acetic acid derivatives.
Biochem/physiol Actions
Zolmitriptan is a selective serotonin receptor agonist of the 5HT1B and 5HT1D subtypes, both centrally and peripherally. It has been used clinically for the acute treatment of migraine attacks with or without aura and cluster headaches.
Pharmacokinetics
Zolmitriptan, like other triptans, is a serotonin (5-hydroxytryptamine; 5-HT) receptor agonist, with enhanced specificity for the 5-HT1B and 5-HT1D receptor subtypes. It is through the downstream effects of 5-HT1B/1D activation that triptans are proposed to provide acute relief of migraines. Zolmitriptan is also a vasoconstrictor, leading to possible adverse cardiovascular effects such as myocardial ischemia/infarction, arrhythmias, cerebral and subarachnoid hemorrhage, stroke, gastrointestinal ischemia, and peripheral vasospastic reactions. In addition, chest/throat/neck/jaw pain, tightness, and/or pressure has been reported, along with the possibility of medication overuse headaches and serotonin syndrome. Patients with phenylketonuria should be advised that ZOMIG-ZMT contains phenylalanine.
Clinical Use
Zomig was launched in Germany, Denmark, Sweden and the UK for use as an antimigraine agent (with and without aura). It can be prepared by three related routes of 5 to 7 steps starting from L-4-nitrophenylalanine. Zomig is a 5-HT1D/1B receptor agonist (10 fold ratio) with modest (< 100x) affinity for 5-HT1A and 5-HT1F receptors. It has no affinity for other serotonin receptors or receptors of other neurotransmitters. It has a novel dual action mechanism: centrally it acts on the trigeminal nucleus caudalis and peripherally is acts on the trigeminovascular system. Zomig was effective in treating headaches and nonheadache (photophobia, phonophobia and nausea) symptoms. It was 2-3 times more potent than sumatriptan and is metabolized to three compounds, one of which is 2-8 times more active than the parent. It caused a 40-50% decrease in headache after 1 h and a 73-77% after 4 h. There was a 30% reoccurance of headache but 90% effective treatment with a second dose. It blocks neurogenic inflammation by inhibiting release of peptides, causes vasoconstriction, and inhibits neuronal depolarization at peripheral sites in the cranium. It is 40% bioavailable and a 10 time theraputic dose showed no safety concerns.
Drug interactions
Potentially hazardous interactions with other drugs
Antibacterials: quinolones possibly inhibit
metabolism - reduce dose of zolmitriptan.
Antidepressants: increased risk of CNS toxicity
with citalopram - avoid; risk of CNS toxicity
with MAOIs and moclobemide - reduce dose
of zolmitriptan to max 7.5 mg; SSRIs inhibit
metabolism of zolmitriptan, reduce dose with
fluvoxamine; possibly increased serotonergic
effects with duloxetine and venlafaxine; increased
serotonergic effects with St John’s wort - avoid
concomitant use.
Cimetidine: inhibits metabolism of zolmitriptan;
maximum dose is 5 mg.
Dapoxetine: possible increased risk of serotonergic
effects - avoid for 2 weeks after stopping 5HT1
agonists.
Ergot alkaloids: increased risk of vasospasm.
Linezolid: risk of CNS toxicity - reduce dose of
zolmitriptan.
Metabolism
Zolmitriptan is metabolized in the liver, and studies using cytochrome P450 inhibitors like cimetidine suggest that it is likely metabolized by CYP1A2, as well as by monoamine oxidase (MAO). Zolmitriptan metabolism results in three major metabolites: an active N-desmethyl metabolite (183C91) as well as inactive N-oxide (1652W92) and indole acetic acid (2161W92) metabolites.
Metabolism
Zolmitriptan is eliminated largely by hepatic biotransformation followed by urinary excretion of the metabolites. There are three major metabolites: the indole acetic acid, (the major metabolite in plasma and urine), the N-oxide and N-desmethyl analogues. Only the N-desmethylated metabolite is active. The primary metabolism of zolmitriptan is mediated mainly by the cytochrome P450 isoenzyme CYP1A2 while monoamine oxidase type A is responsible for further metabolism of the N-desmethyl metabolite. Over 60
% of a dose is excreted in the urine, mainly as the indole acetic acid, and about 30
% appears in the faeces, mainly as unchanged drug.
Storage
+4°C
References
https://www.drugbank.ca/drugs/DB00315
Rothner, A. D., et al. "Zolmitriptan oral tablet in migraine treatment: high placebo responses in adolescents." Headache the Journal of Head & Face Pain 46.1(2006):101.
Hedlund, C, et al. "Zolmitriptan nasal spray in the acute treatment of cluster headache: a meta-analysis of two studies. " Neurology49.9(2009):1315–1323.
Properties of Zolmitriptan
| Melting point: | 136-141°C |
| Boiling point: | 563.3±38.0 °C(Predicted) |
| alpha | D22 -5.79° (c = 0.5 in methanol) |
| Density | 1.217±0.06 g/cm3(Predicted) |
| storage temp. | 15-25°C |
| solubility | Soluble in DMSO at 5mg/ml |
| form | powder |
| pka | 9.64(at 25℃) |
| color | white to beige |
| optical activity | [α]/D -3 to -8°, c = 1 in methanol |
| λmax | 225nm(lit.) |
| Merck | 14,10189 |
| Stability: | Stable for 2 years from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 3 months. |
| CAS DataBase Reference | 139264-17-8(CAS DataBase Reference) |
Safety information for Zolmitriptan
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P501:Dispose of contents/container to..… |
Computed Descriptors for Zolmitriptan
| InChIKey | ULSDMUVEXKOYBU-ZDUSSCGKSA-N |
Zolmitriptan manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 139264-17-8 Zolmitriptan Standard 97%View Details
139264-17-8 Zolmitriptan Standard 97%View Details
139264-17-8 -
 139264-17-8 98%View Details
139264-17-8 98%View Details
139264-17-8 -
 Zolmitriptan 98%View Details
Zolmitriptan 98%View Details
139264-17-8 -
 Zolmitriptan 98%View Details
Zolmitriptan 98%View Details -
 Zolmitriptan CAS 139264-17-8View Details
Zolmitriptan CAS 139264-17-8View Details
139264-17-8 -
 Zolmitriptan 95.00% CAS 139264-17-8View Details
Zolmitriptan 95.00% CAS 139264-17-8View Details
139264-17-8 -
 Zolmitriptan >98% (HPLC) CAS 139264-17-8View Details
Zolmitriptan >98% (HPLC) CAS 139264-17-8View Details
139264-17-8 -
 Zolmitriptan CAS 139264-17-8View Details
Zolmitriptan CAS 139264-17-8View Details
139264-17-8
