Sulfuryl chloride
- CAS NO.:7791-25-5
- Empirical Formula: Cl2O2S
- Molecular Weight: 134.97
- MDL number: MFCD00011451
- EINECS: 232-245-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
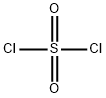
What is Sulfuryl chloride?
Chemical properties
colourless or pale yellow liquid
Chemical properties
A colorless to light yellow fuming liquid. Pungent acrid odor.
Physical properties
Colorless, mobile liquid; turns yellow on standing; very pungent odor; refractive index 1.4437 at 20°C; density 1.667 g/mL at 20°C; vapors heavier than air, vapor density 4.7 (air=1); melts at -51°C; boils at 69.4°C; sparingly soluble in water, decomposing slowly to sulfuric and hydrochloric acids; forms a hydrate SO2Cl2?15H2O with ice-cold water; miscible with benzene, toluene, chloroform, carbon tetrachloride, and glacial acetic acid; decomposed by alkalies (violent reaction occurs).
The Uses of Sulfuryl chloride
Sulfuryl Chloride is a reagent used in the synthesis of catecholic flavonoids as telomerase inhibitors. It is also used to prepare (pyrenebutanamido)thiourea derivs. for anion PET chemosensors.
The Uses of Sulfuryl chloride
Chlorinating and sulfonating or chlorosulfonating agent in organic syntheses, e.g., in the manufacture of chlorophenol and chlorothymol. Has been used in war gas formulations.
Definition
ChEBI: Sulfuryl dichloride is a sulfuryl halide.
General Description
A colorless fuming liquid with a pungent odor. Very toxic by inhalation. Corrosive to metals and tissue.
Reactivity Profile
Sulfuryl chloride reacts exothermically with water. Incompatible with strong oxidizing agents, alcohols, amines. Reacts violently with bases. Attacks many metals. Can react explosively with lead dioxide [Mellor 10:676 1946-47]. May react vigorously or explosively if mixed with diisopropyl ether or other ethers in the presence of trace amounts of metal salts [J. Haz. Mat., 1981, 4, 291].
Hazard
Strong irritant to tissue.
Health Hazard
Vapors cause severe irritation of eyes and respiratory system. Liquid burns eyes and skin. If ingested, can cause severe burns of mouth and stomach.
Fire Hazard
Behavior in Fire: Toxic and irritating gases are generated.
Safety Profile
A corrosive irritant to skin, eyes, and mucous membranes. Questionable carcinogen with experimental tumorigenic data. Can explode with Pb02. Will react with water or steam to produce heat and toxic and corrosive fumes. Incompatible with alkalies, diethyl ether, dimethyl sulfoxide,dinitrogen pentaoxide, lead dioxide, phosphorus. When heated to decomposition it emits highly toxic fumes of Cland SOx. See also SULFURIC ACID and HYDROCHLORIC ACID, which are formed upon hydrolysis.
Potential Exposure
Sulfuryl chloride is used to make other chemicals, including chlorophenol and chlorothymol, disinfectants, pharmaceuticals, phosphate insecticides, heterocyclic herbicides, fungicides, dyestuffs, and some plastics; as a solvent and catalyst; as a chlorosulfonating agent in organic synthesis. Also used as a dehydrating agent, as cathode material and in lithium batteries; as a wool treatment to prevent shrinkage.
Shipping
UN1834 Sulfuryl chloride, Hazard Class: 6.1; Labels: 6.1-Poison Inhalation Hazard; 8-Corrosive material; Inhalation Hazard Zone A. STN: 4930260; Sulfuryl chloride.
Incompatibilities
Water and air reactive. When spilled in water, hydrogen chloride, sulfur dioxide and sulfuric acid are produced. Forms corrosive mixture with air (NFPA Fire Rating: 0). Reacts exothermically with moisture in air, water or steam, releasing heat and yielding sulfuric acid and HCl vapors. Reacts violently with bases, amines, amides, inorganic hydroxides; alkalis, alkali metals, dimethyl sulfoxide, dinitrogen pentoxide, lead dioxide (explosive reaction); N-methylformamide, red phosphorus. Reacts, possibly violently, with oxidizers, organic substances, strong acids, alcohols, amines, ethers e.g., diethyl ether, diisopropyl ether, especially if trace amounts of metal salts are present); glycols, peroxides. Attacks metals in the presence of moisture, forming flammable hydrogen gas. Acid formed by reaction with water can be neutralized by limestone, lime, or soda ash.
Waste Disposal
Wear nitrile rubber gloves, laboratory coat, eye protection and, if necessary, a SCBA. Cover the spill with a 1:1:1 mixture by weight of sodium carbonate or calcium carbonate, clay cat litter (bentonite) and sand. When the sulfuryl chloride has been absorbed, scoop the mixture into a plastic pail of cold water. Allow to stand for 24 hours. Test the pH of the solution and neutralize if necessary with sodium carbonate. Decant the solution to the drain flushing with 50 times its volume of water. Treat the solid residue as normal refuse.
Properties of Sulfuryl chloride
| Melting point: | -54.1 °C |
| Boiling point: | 69.1 °C |
| Density | 1.667 |
| vapor density | 4.7 (vs air) |
| vapor pressure | 100 mm Hg ( 17.8 °C) |
| refractive index | n |
| Flash point: | 69.1°C |
| storage temp. | Hygroscopic, Room Temperature, under inert atmosphere |
| solubility | Miscible with benzene, toluene, chloroform, ether, carbon tetrachloride and glacial acetic acid. |
| form | Liquid |
| color | Clear colorless to yellow |
| Specific Gravity | 1.670 (20/20℃) |
| Water Solubility | reacts |
| Sensitive | Moisture Sensitive |
| Merck | 14,8980 |
| Exposure limits | ACGIH: TWA 50 ppm OSHA: TWA 25 ppm; STEL 125 ppm NIOSH: IDLH 2300 ppm |
| Dielectric constant | 9.2(22℃) |
| Stability: | Reacts violently with water. Incompatible with acids, alcohols, bases, metals, amines, moisture. |
| CAS DataBase Reference | 7791-25-5(CAS DataBase Reference) |
| NIST Chemistry Reference | Sulfuryl chloride(7791-25-5) |
| EPA Substance Registry System | Sulfuryl chloride (7791-25-5) |
Safety information for Sulfuryl chloride
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation H330:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Sulfuryl chloride
Sulfuryl chloride manufacturer
AKASH PHARMA EXPORTS
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran


![2-[[(2-ethylphenyl)(2-hydroxyethyl)amino]methyl]-3,3-difluoro-Propanenitrile](https://img.chemicalbook.in/CAS/GIF/2647-14-5.gif)
You may like
-
 Sulfuryl Chloride 99%View Details
Sulfuryl Chloride 99%View Details -
 Sulfuryl chloride CAS 7791-25-5View Details
Sulfuryl chloride CAS 7791-25-5View Details
7791-25-5 -
 Sulfuryl chloride, 98.5% CAS 7791-25-5View Details
Sulfuryl chloride, 98.5% CAS 7791-25-5View Details
7791-25-5 -
 Sulfuryl chloride, 98.5% CAS 7791-25-5View Details
Sulfuryl chloride, 98.5% CAS 7791-25-5View Details
7791-25-5 -
 Sulfuryl chloride CAS 7791-25-5View Details
Sulfuryl chloride CAS 7791-25-5View Details
7791-25-5 -
 Sulphuryl chloride CAS 7791-25-5View Details
Sulphuryl chloride CAS 7791-25-5View Details
7791-25-5 -
 Sulfuryl Chloride 97% CAS 7791-25-5View Details
Sulfuryl Chloride 97% CAS 7791-25-5View Details
7791-25-5 -
 Sulfuryl chloride CAS 7791-25-5View Details
Sulfuryl chloride CAS 7791-25-5View Details
7791-25-5
