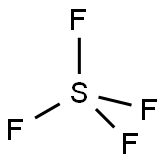
SULFURYL FLUORIDE
- CAS NO.:2699-79-8
- Empirical Formula: F2O2S
- Molecular Weight: 102.06
- MDL number: MFCD00153272
- EINECS: 220-281-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-07-04 15:08:58
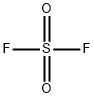
What is SULFURYL FLUORIDE?
Description
Sulfuryl fluoride was first reported by M. Trautz and K. Ehrmann in 1935. It is relatively unreactive for a sulfur–fluorine compound, but it is very irritating to the respiratory tract. It is primarily used as a fumigant, especially for ridding structures of termites. SO2F2 is 4,000 times more powerful as a greenhouse gas than CO2, and it was recently shown that its persistence in the atmosphere is much longer than previously suspected.
Chemical properties
colourless odourless gas
Chemical properties
Sulfuryl fluoride is a colorless, poisonous gas. Odorless.
The Uses of SULFURYL FLUORIDE
Sulfuryl fluoride is another promising chemical, which is widely used for control of termites, and stored-product insects. This chemical shows considerable promise and is undergoing registration procedures as replacement for methyl bromide in several countries including the United States, Great Britain, Italy, and France. The fumigant has vapour pressure of about ten times higher (13,442 mm of Hg at 25oC) than that of methyl bromide and hence it is more penetrative into treated commodities which is a significant benefit in situations where insect infestations are located deep within cracks or machinery in premises such as flour mills . The fumigant has several other advantages as a replacement for methyl bromide; it is nonreactive with structural materials and components of electronic equipment, is non flammable and has not caused problems of taint following treatment of a wide range of materials. However the sulfuryl fluoride was found to be less effective in controlling the insect eggs.
The Uses of SULFURYL FLUORIDE
Fumigant insecticide; termiticide.
The Uses of SULFURYL FLUORIDE
Insect fumigant
Definition
ChEBI: Sulfuryl difluoride is a sulfuryl halide. It has a role as a fumigant insecticide.
General Description
A colorless odorless gas. Shipped as a liquefied gas under its own vapor pressure. Noncombustible. Heavier than air. Very toxic by inhalation. Contact with the unconfined liquid can cause frostbite. Prolonged exposure to heat can cause containers to rupture violently and rocket.
Air & Water Reactions
Reacts with moist air to give corrosive acid mists that are heavier than air. Decomposes with water forming hydrofluoric acid and sulfuric acid. Reacts violently with bases [Handling Chemicals Safely p. 881 1980].
Reactivity Profile
SULFURYL FLUORIDE is incompatible with water, strong oxidizing agents, alcohols, amines, and bases. May react vigorously or explosively if mixed with diisopropyl ether or other ethers in the presence of trace amounts of metal salts [J. Haz. Mat., 1981, 4, 291].
Hazard
Toxic by inhalation. Central nervous system impairment.
Health Hazard
TOXIC; may be fatal if inhaled or absorbed through skin. Vapors may be irritating. Contact with gas or liquefied gas may cause burns, severe injury and/or frostbite. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control may cause pollution.
Fire Hazard
Some may burn but none ignite readily. Vapors from liquefied gas are initially heavier than air and spread along ground. Cylinders exposed to fire may vent and release toxic and/or corrosive gas through pressure relief devices. Containers may explode when heated. Ruptured cylinders may rocket.
Agricultural Uses
Fumigant: Sulfuryl fluoride is used to fumigate closed structures and their contents such as domestic dwellings, garages, barns, storage buildings, commercial warehouses, ships in port, and railroad cars. It controls numerous insect pests including termites, powder post beetles, old house borers, bedbugs, carpet beetles, clothes moths and cockroaches, as well as rats and mice. It is also used in organic synthesis of drugs and dyes. Use pending in EU countries[ 115]. Registered for use in the U.S.
Trade name
TERMAFUME®; VIKANE®; VIKANE FUMIGANT®
Safety Profile
Poison by ingestion. Mildly toxic by inhalation. Accidental human exposure caused nausea, vomiting, cramps, itching. May be narcotic in high concentration. Experimental reproductive effects. Can react with water, steam. When heated to decomposition it emits very toxic fumes of Fand SOx. See also FLUORIDES.
Potential Exposure
Sulfuryl fluoride is used an insecticidal fumigant. It is also used in organic synthesis of drugs and dyes.
Shipping
UN2191 Sulfuryl fluoride, Hazard Class: 2.3; Labels: 2.3-Poisonous gas, Inhalation Hazard Zone D.
Incompatibilities
Reacts with water and steam; at temperatures >300℃, forms strong, highly corrosive acids. Incompatible with strong oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from metal hydroxides, alcohols, alkaline materials, amines, strong acids, strong bases. Fluorides form explosive gases on contact with strong acids or acid fumes.
Waste Disposal
Return refillable compressed gas cylinders to supplier. Addition of soda ash-slaked lime solution to form the corresponding sodium and calcium salt solution. This solution can be safely discharged after dilution. The precipitated calcium fluoride may be buried or added to a landfill. Small amounts could also be released directly to the atmosphere without serious harm.
Properties of SULFURYL FLUORIDE
| Melting point: | -121,4°C |
| Boiling point: | -55,2°C |
| Density | gas: 4.55g/L; liq; 1.7 [CRC10] |
| solubility | slightly soluble in H2O, ethanol; soluble in toluene,ctc |
| form | colorless gas |
| color | colorless |
| Water Solubility | mL SO2F2/100mL solvent: 4–5, H2O; 24–27, alcohol; 210–220, toluene; 136–138, CCl4, [MER06] |
| Stability: | Stable. Reacts with water. |
| CAS DataBase Reference | 2699-79-8(CAS DataBase Reference) |
| EPA Substance Registry System | Sulfuryl fluoride (2699-79-8) |
Safety information for SULFURYL FLUORIDE
Computed Descriptors for SULFURYL FLUORIDE
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 1-methylindoline-2,3-dione 98%View Details
1-methylindoline-2,3-dione 98%View Details
2058-74-4 -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
