Zinc tetrafluoroborate
- CAS NO.:13826-88-5
- Empirical Formula: B2F8Zn
- Molecular Weight: 239
- MDL number: MFCD00049619
- EINECS: 237-534-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 13:37:16
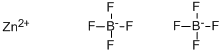
What is Zinc tetrafluoroborate?
Chemical properties
clear colorless solution
The Uses of Zinc tetrafluoroborate
Plating and bonderizing, resin curing.
What are the applications of Application
Zinc tetrafluoroborate is a starting material for homometallic, trinuclear heteroscorpionate complexes
General Description
Colorless odorless aqueous solution. Sinks and mixes with water.
Reactivity Profile
Acidic inorganic salts, such as Zinc tetrafluoroborate, are generally soluble in water. The resulting solutions contain moderate concentrations of hydrogen ions and have pH's of less than 7.0. They react as acids to neutralize bases. These neutralizations generate heat, but less or far less than is generated by neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible. Many of these compounds catalyze organic reactions.
Health Hazard
Ingestion may cause irritation or corrosion of the alimentary tract. Contact with eyes or skin causes irritation.
Properties of Zinc tetrafluoroborate
| Density | 1.43 |
| form | crystal |
| color | white |
| CAS DataBase Reference | 13826-88-5(CAS DataBase Reference) |
| EPA Substance Registry System | Borate(1-), tetrafluoro-, zinc (2:1) (13826-88-5) |
Safety information for Zinc tetrafluoroborate
Computed Descriptors for Zinc tetrafluoroborate
Zinc tetrafluoroborate manufacturer
Jayfluoride PVT LTD
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
 13826-88-5 Zinc tetrafluoroborate 98%View Details
13826-88-5 Zinc tetrafluoroborate 98%View Details
13826-88-5 -
 Zinc Tetrafluoroborate CASView Details
Zinc Tetrafluoroborate CASView Details -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1