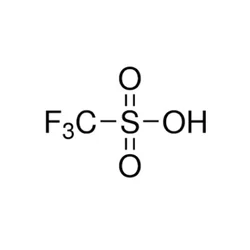
Trifluoromethanesulfonic acid
Synonym(s):TFMSA;Triflic acid;Triflic acid, TFMS;Trifluoromethanesulfonic acid
- CAS NO.:1493-13-6
- Empirical Formula: CHF3O3S
- Molecular Weight: 150.08
- MDL number: MFCD00007514
- EINECS: 216-087-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-11-18 17:49:23
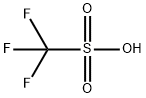
What is Trifluoromethanesulfonic acid?
Chemical properties
Clear very light yellow liquid. Smoke in the air, easy to absorb water to form a hydrate. It is easily soluble in water, releases a lot of heat, and hydrolyzes to generate trifluoromethane (CHF3) and sulfuric acid.
The Uses of Trifluoromethanesulfonic acid
Trifluoromethanesulfonic acid acts as a catalyst for esterification reactions and an acidic titrant in nonaqueous acid-base titration. It is useful in protonations due to the presence of conjugate base triflate is non nucleophilic. It serves as a deglycosylation agent for glycoproteins. In addition, it is a precursor and a catalyst in organic chemistry. It reacts with acyl halides to prepare mixed triflate anhydrides, which are strong acylating agents used in Friedel-Crafts reactions. It acts as a key starting material for the preparation of ethers and olefins by reacting with alcohols as well as to prepare trifluoromethanesulfonic anhydride by dehydration reaction.
The Uses of Trifluoromethanesulfonic acid
As a catalyst in Friedel-Crafts type acylation, alkylation and polymerization reactions; as a solvent for ESR; as a nonaqueous strong acid titrant; with trifluoroacetic acid, q.v., in solid-phase peptide synthesis. One of the strongest available monoprotic acids.
What are the applications of Application
Trifluoromethanesulfonic acid is a strong monoprotic acid
Definition
ChEBI: Trifluoromethanesulfonic acid is a one-carbon compound that is methanesulfonic acid in which the hydrogens attached to the methyl carbon have been replaced by fluorines. It is a one-carbon compound and a perfluoroalkanesulfonic acid. It is a conjugate acid of a triflate.
General Description
Trifluoromethanesulfonic acid is a strong organic acid. It can be prepared by reacting bis(trifluoromethylthio)mercury with H2O2. On mixing with HNO3, it affords a nitrating reagent (a nitronium salt). This reagent is useful for the nitration of aromatic compounds. Its dissociation in various organic solvents has been studied.
Safety Profile
A corrosive irritant to the skin, eyes, and mucous membranes. A strong acid. Violent reaction with acyl chlorides or aromatic hydrocarbons evolves toxic hydrogen chloride gas. When heated to decomposition it emits toxic fumes of Fand SOx. See also FLUORIDES.
Properties of Trifluoromethanesulfonic acid
| Melting point: | -40 °C |
| Boiling point: | 162 °C (lit.) |
| Density | 1.696 g/mL at 25 °C (lit.) |
| vapor density | 5.2 (vs air) |
| vapor pressure | 8 mm Hg ( 25 °C) |
| refractive index | n |
| RTECS | PB2771000 |
| Flash point: | None |
| storage temp. | Store below +30°C. |
| solubility | Miscible in H<sub>2</sub>O |
| form | Fuming Liquid |
| appearance | Colorless liquid |
| pka | -14(at 25℃) |
| Specific Gravity | 1.696 |
| color | slightly brown |
| PH | <1 (H2O) |
| Water Solubility | SOLUBLE |
| Sensitive | Hygroscopic |
| Merck | 14,9676 |
| BRN | 1812100 |
| Stability: | Stable. Incompatible with acids, alkalis, metals. |
| CAS DataBase Reference | 1493-13-6(CAS DataBase Reference) |
| NIST Chemistry Reference | CF3SO3H(1493-13-6) |
| EPA Substance Registry System | Methanesulfonic acid, trifluoro- (1493-13-6) |
Safety information for Trifluoromethanesulfonic acid
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H290:Corrosive to Metals H302:Acute toxicity,oral H314:Skin corrosion/irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P234:Keep only in original container. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Trifluoromethanesulfonic acid
| InChIKey | ITMCEJHCFYSIIV-UHFFFAOYSA-N |
Trifluoromethanesulfonic acid manufacturer
JSK Chemicals
Sri Haimavathi Organics
SRF LIMITED
ASM Organics
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 Trifluoromethanesulfonic acid supplierView Details
Trifluoromethanesulfonic acid supplierView Details
1493-13-6 -
 TRIFLIC ACID 99%View Details
TRIFLIC ACID 99%View Details -
 Trifluoromethanesulfonic acid 98%View Details
Trifluoromethanesulfonic acid 98%View Details -
 Trifluoromethanesulfonic acid CAS 1493-13-6View Details
Trifluoromethanesulfonic acid CAS 1493-13-6View Details
1493-13-6 -
 Triflic AcidView Details
Triflic AcidView Details
1493-13-6 -
 Triflic AcidView Details
Triflic AcidView Details
1493-13-6 -
 Liquid Trifluoromethane Sulfonic Acid, For Laboratory, Grade Standard: Reagent GradeView Details
Liquid Trifluoromethane Sulfonic Acid, For Laboratory, Grade Standard: Reagent GradeView Details
1493-13-6 -
 TRIFLUROMETHANESULFONIC ACID, 25kg BagView Details
TRIFLUROMETHANESULFONIC ACID, 25kg BagView Details
1493-13-6
