Ammonium hydrogen difluoride
Synonym(s):Ammonium bifluoride;Ammonium Hydrogen Fluoride;Etching powder
- CAS NO.:1341-49-7
- Empirical Formula: F2H5N
- Molecular Weight: 57.04
- MDL number: MFCD00012018
- EINECS: 215-676-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-18 17:17:19
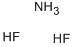
What is Ammonium hydrogen difluoride?
Chemical properties
white scales or flakes, also referred to asammonium hydrogen fluoride, anunonium difluoride and anunonium acid fluoride.
Physical properties
Orthorhombic or tetragonal crystals; etches glass; deliquescent; density 1.50 g/cm3; refractive index 1.390; melts at 125.6°C; very soluble in water; slightly soluble in alcohol.
The Uses of Ammonium hydrogen difluoride
In manufacture of Mg and Mg alloys; in brightening of Al; for purifying and cleansing various parts of beer-dispensing apparatus, tubes, etc., sterilizing dairy and other food equipment; in glass and porcelain industries; as mordant for aluminum; as a "sour" in laundering cloth. In lab production of HF.
The Uses of Ammonium hydrogen difluoride
Ammonium hydrogen fluoride is used as a chemical reagent in analysis and an antiseptic. It is also used in ceramics, electroplating, component of glass etchants as well as food processing equipment disinfectant. It acts as an intermediate in the production of hydrofluoric acid from hexafluorosilicic acid.
General Description
Ammonium hydrogen difluoride (NH4HF2) is usually used to induce fluorination of mineral raw materials to modify the mechanical properties and solubility.Compared with many other fluoride sources, NH4HF2 has the advantages of good solubility and low cost.
Air & Water Reactions
Dissolves in water and forms a weak solution of hydrofluoric acid.
Reactivity Profile
AMMONIUM BIFLUORIDE reacts violently with bases. In presence of moisture will corrode glass, cement, and most metals. Flammable hydrogen gas may collect in enclosed spaces. Do not use steel, nickel, or aluminum containers [USCG, 1999].
Hazard
Corrosive to skin.
Health Hazard
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
Flammability and Explosibility
Non flammable
Safety Profile
Caustic poison and strong irritant by all routes. See also HYDROFLUORIC ACID. When heated to decomposition it emits very toxic fumes of F-, NO,, and NH3.
Potential Exposure
It is used as a sterilizer, in dairy and brewery operations; in the ceramic, glass, and electroplating industries; as a laundry sour.
Shipping
Administer oxygen if breathing is difficult. Remove and isolate contaminated clothing and shoes. In case of contact with substance, immediately flush skin or eyes with running water for at least 20 minutes. For minor skin contact, avoid spreading material on unaffected skin. Keep victim warm and quiet. Effects of exposure (inhalation, ingestion or skin contact) to substance may be delayed. Ensure that medical personnel are aware of the material(s) involved and take precautions to protect themselves. Medical observation is recommended for 24 to 48 hours after breathing overexposure, as pulmonary edema may be delayed. As first aid for pulmonary edema, a doctor or authorized paramedic may consider administering a drug or other inhalation therapy.
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. In the presence of moisture corrodes concrete, metals, glass.
Waste Disposal
May be buried in a specially designated chemical landfill. Aqueous wastes may be reacted with an excess of lime followed by lagooning and either recovery or land disposal of the separated calcium fluoride.
Properties of Ammonium hydrogen difluoride
| Melting point: | 125 °C(lit.) |
| Boiling point: | 230 °C |
| Density | 1,5 g/cm3 |
| vapor pressure | 1 hPa (20 °C) |
| Flash point: | 238°C |
| storage temp. | Store at room temperature. |
| solubility | 630g/l |
| form | Solid |
| pka | 6.3[at 20 ℃] |
| Specific Gravity | 1.5 |
| color | White |
| PH | 2 (5.7g/l, H2O, 20℃) |
| Odor | acidic odor |
| Water Solubility | 630 g/L (20 ºC) |
| Sensitive | Hygroscopic |
| Merck | 14,498 |
| Exposure limits | ACGIH: TWA 2.5 mg/m3 NIOSH: IDLH 250 mg/m3; TWA 2.5 mg/m3 |
| Stability: | hygroscopic |
| CAS DataBase Reference | 1341-49-7(CAS DataBase Reference) |
| EPA Substance Registry System | Ammonium bifluoride (1341-49-7) |
Safety information for Ammonium hydrogen difluoride
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Ammonium hydrogen difluoride
| InChIKey | KVBCYCWRDBDGBG-UHFFFAOYSA-N |
Ammonium hydrogen difluoride manufacturer
Madan Minerals & Chemicals Industries
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
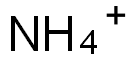
You may like
-
 AMMONIUM BI FLUORIDE 99%View Details
AMMONIUM BI FLUORIDE 99%View Details -
 Ammonium Bi Fluoride 99%View Details
Ammonium Bi Fluoride 99%View Details -
 Ammonium hydrogen fluoride CAS 1341-49-7View Details
Ammonium hydrogen fluoride CAS 1341-49-7View Details
1341-49-7 -
 Ammonium hydrogen fluoride CAS 1341-49-7View Details
Ammonium hydrogen fluoride CAS 1341-49-7View Details
1341-49-7 -
 Ammonium Bi Fluoride CASView Details
Ammonium Bi Fluoride CASView Details -
 Ammonium Bifluoride Powder, 50kg BagView Details
Ammonium Bifluoride Powder, 50kg BagView Details
1341-49-7 -
 Crystals Ammonium Bifluoride PureView Details
Crystals Ammonium Bifluoride PureView Details
1341-49-7 -
 Ammonium BifluorideView Details
Ammonium BifluorideView Details
1341-49-7
