Sodium fluoride
Synonym(s):Sodium fluoride;Sodium fluoride solution;Sodium monofluoride;Sodium monofluoride (NaF);Disodium Fluoride
- CAS NO.:7681-49-4
- Empirical Formula: FNa
- Molecular Weight: 41.99
- MDL number: MFCD00003524
- EINECS: 231-667-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:50:35
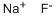
What is Sodium fluoride?
Absorption
Sodium fluoride is 90% absorbed from the gastrointestinal tract, with 77% of absorption in the proximal intestine and about 25% in the stomach. The rate of absorption may vary according to gastric pH. Cmax is reached 20-60 minutes after ingestion. Cmax was estimated to be 848 ± 116 ng/mL after a 20mg sodium fluoride solution was ingested, with a Tmax of 0.46 ± 0.17 hours. The bioavailability of sodium fluoride tablets administered in the fasted state during one pharmacokinetic study approached 100%. Another resource reports a sodium fluoride AUC of 1.14 ± 0.12 μg × h/mL after the ingestion of fluoridated water.
Toxicity
The oral LD50 of sodium fluoride is 44 mg/kg in mice and 31 mg/kg in rats. The oral LD50 of sodium fluoride in rabbits is 200 mg/kg.
Overdose information
The ingestion of toothpaste is the major cause of sodium fluoride overdose. This is followed by sodium fluoride supplements and mouth rinses. Most causes of sodium fluoride toxicity have been observed in children under the age of 6 years old. The manifestations of a sodium fluoride overdose may include gastrointestinal disturbance, abdominal pain, alterations in taste, seizures, salivation, bradycardia, tachycardia, headache, tremor, and shallow breathing. Gastrointestinal bleeding may also occur in addition to a sensation of burning in the mouth. Hypotension, bronchospasm, fixed mydriasis, and elevated potassium can also occur which, in turn, may lead to arrhythmias and cardiac arrest.
Management
If a dose greater than 5 mg fluoride per kilogram of body weight (2.3 mg fluoride per pound of body weight) has been taken, it is advisable to induce vomiting. Administer calcium in an oral, soluble form (for example, 5% calcium gluconate, a solution of calcium lactate, or milk). The patient should seek immediate medical attention. If a sodium fluoride ingestion of 15 mg fluoride/kg of body weight or more occurs (i.e. higher than 6.9 mg fluoride per pound), immediately induce vomiting, provide supportive care, and admit the patient to the hospital for observation.
Description
Sodium fluoride, NaF, is a binary salt that is a clear, lustrous crystal or white powder. The insecticide grade is frequently dyed blue. It is soluble in water and has a specific gravity of 2.558, which is heavier than water. Sodium fluoride is highly toxic by ingestion and inhalation, and is also strongly irritating to tissue. The TLV is 2.5 mg/m3 of air. The four-digit UN identification number is 1690. The primary uses are fluoridation of municipal water at 1 ppm, as an insecticide, rodenticide, and fungicide, and in toothpastes and disinfectants.
Description
Sodium fluoride has replaced stannous fluoride and sodium monofluorophosphate as the most commonly used cavity-fighting agent in commercial toothpastes, although it didn''t make its commercial debut until 1982. It works well with the kinds of silica abrasives (polishing agents) used in translucent gel toothpastes. Sodium fluoride is a close chemical relative of sodium chloride, ordinary table salt.
Chemical properties
White or almost white powder or colourless crystals.
Chemical properties
Sodium fluoride is a white powder or colorless crystals. Often used in a solution. Odorless. Pesticide grade is often dyed blue.
Physical properties
Colorless cubic or tetragonal crystals; density 2.78 g/cm3; melts at 993°C; vaporizes at 1,695°C; moderately soluble in water 4.22 g/100mL at 18°C; soluble in hydrofluoric acid; insoluble in ethanol.
The Uses of Sodium fluoride
Sodium fluoride (NaF), in the concentration of one ppm, is added to municipal drinking water to help reduce tooth decay. It is also used as an insecticide, fungicide, and rodenticide, as well as in the manufacture of adhesives, disinfectants, and dental products.
The Uses of Sodium fluoride
As insecticide, particularly for roaches and ants; in other pesticide formulations; constituent of vitreous enamel and glass mixes; as a steel degassing agent; in electroplating; in fluxes; in heat-treating salt compositions; in the fluoridation of drinking water; for disinfecting fermentation apparatus in breweries and distilleries; preserving wood, pastes and mucilage; manufacture of coated paper; frosting glass; in removal of HF from exhaust gases to reduce air pollution. Dental caries prophylactic.
The Uses of Sodium fluoride
Sodium fluoride, formed by reaction of sodium carbonate and hydrofluoric acid, and then evaporating. Used (1) as an antiseptic and antifermentative in alcohol distilleries, (2) as a food preservative, (3) as a poison for rats and roaches, (4) as a constituent of ceramic enamels and fluxes; sodium hydrogen fluoride, sodium difluoride, sodium acid fluoride, NaHF2, white solid, soluble, formed by reaction of sodium carbonate and excess hydrofluoric acid, and then evaporating. Used (1) as an antiseptic, (2) for etching glass, (3) as a food preservative, (4) for preserving zoological specimens.
What are the applications of Application
Sodium Fluoride is an inhibitor of serine/threonine phosphatases widely used in bone and dental research
Indications
Sodium fluoride in the oral or topical form is indicated for the prevention and control of dental caries and for the maintenance of dental health. Fluoride supplements in the form of tablets and other formulas may be prescribed to prevent tooth decay in high-risk children aged 6 months to 16 years old whose drinking water source contains low fluoride concentrations.
Background
Sodium fluoride is an inorganic chemical compound that is a source of the fluoride ion in many applications, including dental care and radiographic imaging when it is used as Fluoride ion F-18. Sodium fluoride's benefits on dental health were first observed in the 1930s, when individuals in communities with fluoridated drinking water showed less tooth decay than those without fluoridated water. The use of fluoride in dental practice began in the 1940s. Now, sodium fluoride may be found in a variety of gels, varnishes, rinses, toothpaste products, and fluoride treatments provided in dental care. According to the American Dental Association (ADA), thorough evidence reviews have indicated that the use of fluoride to prevent and control dental caries is safe when used correctly and is highly effective in reducing the prevalence of caries.
Definition
ChEBI: A metal fluoride salt with a Na(+) counterion.
Preparation
Sodium fluoride is prepared by adding sodium hydroxide or sodium carbonate to a 40% solution of hydrofluoric acid. In excess hydrofluoric acid, sodium bifluoride, NaHF2 , is formed. NaF also is made by fusion of cryolite with caustic soda. Technical grade products are usually sold at 90 to 95% purity.
brand name
Fluorinse (Oral-B); Minute-Gel (Oral-B); Neutra Care (Oral-B); Pediaflor (Ross).
General Description
Sodium fluoride is a colorless crystalline solid or white powder, or the solid dissolved in a liquid. Sodium fluoride is formed by the reaction of sodium carbonate and hydrofluoric acid. Sodium fluoride is soluble in water. Sodium fluoride is noncombustible. Sodium fluoride is corrosive to aluminum. Sodium fluoride is used as an insecticide. Sodium fluoride is also used to fluorinate water supplies, as a wood preservative, in cleaning compounds, manufacture of glass, and for many other uses.
Hazard
Toxic by ingestion and inhalation, strong irritant to tissue.
Health Hazard
Ingestion may cause vomiting, abdominal pain, diarrhea, convulsions, collapse, thirst, disturbed color vision, acute toxic nephritis.
Health Hazard
The acute toxicity of fluorides is generally moderate. High exposures may cause irritation of the eyes and respiratory tract. Ingestion of fluoride may cause a salty or soapy taste, vomiting, abdominal pain, diarrhea, shortness of breath, difficulty in speaking, thirst, weak pulse, disturbed color vision, muscular weakness, convulsions, loss of consciousness, and death. In humans the approximate lethal dose of NaF by ingestion is 5 g. Repeated inhalation of fluoride dust may cause excessive calcification of the bone and calcification of ligaments of the ribs, pelvis, and spinal column. Repeated skin contact may cause a rash Fluorides have not been shown to be carcinogenic or to show reproductive or developmental toxicity in humans.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
Fire Hazard
Fluorides are not combustible.
Flammability and Explosibility
Fluorides are not combustible.
Industrial uses
Sodium fluoride is a white to yellowish powder, poorly soluble in water. The NaF solubility in water is 3.85% at 15 °C and 4.21% at 25 °C. Commercially, NaF is obtained by reacting hydrofluoric acid with sodium hydroxide. This reaction is a by-product during the production of superphosphates from fluoro-apatite. Sodium fluoride is an important depressant used exclusively during beneficiation of non-sulfide minerals, as well as the rare-earth minerals as a depressant alone or in combination with other depressants. It is used together with starch as co-depressant for rutile and ilmenite during zirconium– titanium separation or during reverse silicate flotation from rutile and ilmenite with cationic collectors. Although it is believed that Na2F is a silicate depressant, studies have shown that it does not depress silicate minerals. In fact, it improves depression of oxides and silicates when used with other depressants.
Biochem/physiol Actions
Sodium fluoride (NaF) prevents dental caries, by catalysing the diffusion of calcium and phosphate and remineralizes the lesions. Fluoride affects spermatogenesis and capacitation process in the female reproductive tract. Fluoride induces oxidative stress, DNA damage in ovary ultimately affects the development of oocytes. Fluoride supplementation enhances bone mineral density. 18F-NaF is a radiotracer used in positron emission tomography (PET) to identify atherosclerotic lesions and other cardiac plaques.
Pharmacokinetics
Sodium fluoride protects the teeth from acid demineralization while preventing tooth decay by bacteria while strengthening tooth enamel. It is important to note that excess fluoride exposure during tooth mineralization, especially in children 1-3 years old, may cause fluorosis. It is a condition manifested by white lines, pitting, or discoloration of teeth resulting from changes in tooth enamel. The risk of fluorosis can be decreased by the use of a rice-size amount of fluoridated toothpaste in children younger than 3 years old. It is recommended that no more than a pea-sized quantity of fluoridated toothpaste should be used for children from 3 to 6 years old. The American Dentistry Association (ADA) recommends that children should be closely supervised during toothpaste use to prevent excess fluoride ingestion.
Clinical Use
Sodium fluoride (NaF) promotes the proliferation and activity of osteoblasts and is classified as a nonhormonal bone-forming agent. Because treatment with NaF induces bone formation, it is essential that this therapy be coupled with oral calcium supplementation (1,000 mg/day). Additionally, NaF exhibits moderate antiresorptive activity, because it inhibits osteoclastic activity when it is absorbed into the bone matrix. In the treatment of osteoporosis, the therapeutic window for this agent is fairly narrow: Doses less than 45 mg/day are subtherapeutic, and doses in excess of 75 mg/day impair bone mineralization. In addition, the bone that is formed in the presence of NaF is neither as well mineralized nor as strong as normal bone tissue. In fact, some
Safety Profile
Human poison by ingestion. Experimental poison by ingestion, skin contact, intravenous, intraperitoneal, subcutaneous, and intramuscular routes. Human systemic effects: changes in teeth and supporting structures, cyanosis, diarrhea, EKG changes, fluid intake, headache, hypermotihty, increased immune response, muscle weakness, musculo-skeletal changes, nausea or vomiting, paresthesia, ptosis (drooping of the eyelid from sympathetic innervation), respiratory depression, salivary gland changes, tremors. Experimental teratogenic and reproductive effects. Human mutation data reported. A corrosive irritant to skin, eyes, and mucous membranes. Questionable carcinogen with experimental tumorigenic data. It is very phytotoxic. When heated to decomposition it emits toxic fumes of Fand NazO. Used in chemical cleaning, for fluoridation of drinking water, as a funglcide and insecticide. See also FLUORIDES
Potential Exposure
Widely used in the chemical industry; in water treatment and fluoridation of drinking water; as an insecticide, fungicide, and rodenticide; chemical cleaning; electroplating, glass manufacture; vitreous enamels; preservative for adhesives; toothpastes, disinfectant, dental prophylaxis; also used orally in the treatment of various bone diseases to increase bone density and to relieve bone pain.
Metabolism
Several factors can affect the metabolism of sodium fluoride. These include disorders of acid-base balance, circadian rhythm, hematocrit level, high or low altitude, the level physical activity, hormonal status, renal function, genetic predispositions in addition to the diet.
Shipping
UN1690 Sodium fluoride, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Purification Methods
Crystallise NaF from water by partial evaporation in a vacuum desiccator, or dissolve it in water, and precipitate ca half of it by adding EtOH. The precipitate is dried in an air oven at 130o for one day, and then stored in a desiccator over KOH. Its solubility in H2O is 4% at 15o and 4.3% at 25o. [Kwasnik in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 235 1963].
Incompatibilities
Contact with acids release toxic gas. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides.
Waste Disposal
In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office.
Properties of Sodium fluoride
| Melting point: | 993 °C (lit.) |
| Boiling point: | 1700 °C |
| Density | 1.02 g/mL at 20 °C |
| vapor pressure | 1.4 mm Hg ( 0 °C) |
| refractive index | 1.336 |
| Flash point: | 1704°C |
| storage temp. | 2-8°C |
| solubility | H2O: 0.5 M at 20 °C, clear, colorless |
| form | powder |
| color | White to off-white |
| Specific Gravity | 2.558 |
| PH | 7.0-10.0 (25℃, 0.5M in H2O) |
| Odor | Odorless |
| Water Solubility | 4 g/100 mL (25 ºC) |
| Sensitive | Hygroscopic |
| Merck | 14,8618 |
| Exposure limits | ACGIH: TWA 2.5 mg/m3 NIOSH: IDLH 250 mg/m3; TWA 2.5 mg/m3 |
| Stability: | Stable. Hydrolyzed by water. Reacts with mineral acids to generate highly toxic hydrogen fluoride. Incompatible with glass. |
| CAS DataBase Reference | 7681-49-4(CAS DataBase Reference) |
| NIST Chemistry Reference | Sodium fluoride(7681-49-4) |
| EPA Substance Registry System | Sodium fluoride (7681-49-4) |
Safety information for Sodium fluoride
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Sodium fluoride
Sodium fluoride manufacturer
JSK Chemicals
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 Sodium Fluoride 99%View Details
Sodium Fluoride 99%View Details -
 Sodium fluoride 98%View Details
Sodium fluoride 98%View Details -
 Sodium fluoride 98%View Details
Sodium fluoride 98%View Details -
 Fluoride CAS 7681-49-4View Details
Fluoride CAS 7681-49-4View Details
7681-49-4 -
 Fluoride CAS 7681-49-4View Details
Fluoride CAS 7681-49-4View Details
7681-49-4 -
 Sodium fluoride, 99% 99%View Details
Sodium fluoride, 99% 99%View Details -
 Sodium Formate pure CAS 141-33-7View Details
Sodium Formate pure CAS 141-33-7View Details
141-33-7 -
 Sodium Fluoride IP/USPView Details
Sodium Fluoride IP/USPView Details
7681-49-4
