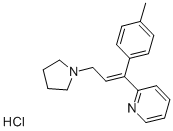
TRIPROLIDINE HYDROCHLORIDE
- CAS NO.:550-70-9
- Empirical Formula: C19H23ClN2
- Molecular Weight: 314.85
- MDL number: MFCD00039044
- EINECS: 208-985-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
What is TRIPROLIDINE HYDROCHLORIDE?
Originator
Actidil,Burroughs-Wellcome,US,1958
The Uses of TRIPROLIDINE HYDROCHLORIDE
trans-Triprolidine Hydrochloride is a histamine H1 receptor antagonist, which can be used for the prevention of treatment of chronic muscle pain.
The Uses of TRIPROLIDINE HYDROCHLORIDE
Triprolidine is an antihistaminic agent with anticholinergic properties. Triprolidine is used to treat and prevent symptoms associated with allergies. Triprolidine is also used in combination with cold medicine to provide relief for flu-like symptoms.
The Uses of TRIPROLIDINE HYDROCHLORIDE
Antihistaminic;H1 antagonist
Definition
ChEBI: A hydrochloride resulting from the formal reaction of equimolar amounts of triprolidine and hydrogen chloride. Its monohydrate is used for the symptomatic relief of uticaria, rhinitis, and various pruritic skin disorders.
Manufacturing Process
4-Methylacetophenone is first reacted with paraformaldehyde and then with
pyrrolidine to give p-methyl-ω-pyrralidinopropiophenone.
Atomized lithium (26 g, 3.75 mols) and sodium-dried ether (200 cc) are
placed in a 3-liter, 3-necked flask fitted with a Herschberg stirrer,
thermometer pocket and a water condenser closed by a calcium chloride tube.
A slow stream of dry nitrogen is blown through the flask, which is cooled to -
10°C and n-butyl chloride (138 g, 156 cc, 1.5 mols) is run in with rapid
stirring; the mixture is stirred for a further 30 minutes, and then cooled to -
60°C
2-Bromopyridine (193 g, 1.22 mols) is then added dropwise over 20 minutes,
the temperature of the reaction mixture being maintained at -50°C. The
mixture is stirred for 10 minutes at -50°C and p-methyl-ω-
pyrrolidinopropiophenone (112.5 g, 0.5 mol) in dry benzene is then added
dropwise over ca 30 minutes, at a temperature of -50°C. The mixture is
stirred for a further 2 hours, the temperature being allowed to rise to -30°C
but no higher.
The mixture is poured onto excess ice, acidified with concentrated
hydrochloric acid, the ether layer separated and extracted with water (1 x 200
cc). The combined aqueous extracts are washed with ether (1 x 200 cc)
basified with 0.880 ammonia and extracted with chloroform (3 x 350 cc); the
extract is washed with water (2 x 100 cc), dried over sodium sulfate,
evaporated, and the residue extracted with boiling light petroleum (BP 60° to
80°C; 10 volumes), filtered hot and evaporated to dryness. The residue is
recrystallized from alcohol to give a cream solid (119 g, 80%), MP 117° to
118°C. Recrystallization gives 1-(4-methylphenyl)-1-(2-pyridyl)-3-
pyrrolidonopropan-1-ol, MP 119° to 120°C.
1-(4-Methylphenyl)-1-(2-pyridyl)-3-pyrrolidinopropan-1-ol (10.0 g) is heated
in a steam bath for 30 minutes with 85% aqueous sulfuric acid (30 cc). The
solution is then poured onto crushed ice, excess of ammonia solution added
and the liberated oil extracted with light petroleum (BP 60° to 80°C). The
extract is dried over anhydrous sodium sulfate and the solvent evaporated to
leave an amber syrup (8.8 g) consisting of the cis and trans isomers of 1-(4-
methylphenyl)-1-(2-pyridyl)-3-pyrrolidinoprop-1-ene as described in US Patent
2,712,023. The isomers may be separated by base exchange chromatography.
The 4-methyl-ω-pyrrolidinopropiophenone required as the starting product for the preparation of the carbinol is prepared by the Mannich reaction (Blicke,
Organic Reactions, 1942, vol 1, p 303; Adamson & Billinghurst, Journal of the
Chemical Society, 1950,1039) from 4-methylacetophenone and pyrrolidine.
The hydrochloride has a MP of 170°C with decomposition.
brand name
Actidil (GlaxoSmithKline); Myidyl (USl).
Therapeutic Function
Antihistaminic
Properties of TRIPROLIDINE HYDROCHLORIDE
| Melting point: | 116-118 °C |
| storage temp. | 2-8°C |
| solubility | Chloroform (Slightly), Dichloromethane (Slightly), Water (Slightly) |
| form | Powder |
| Water Solubility | Soluble to 100 mM in water |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 550-70-9(CAS DataBase Reference) |
| EPA Substance Registry System | Pyridine, 2-[(1E)-1-(4-methylphenyl)-3-(1-pyrrolidinyl)-1-propenyl]-, monohydrochloride (550-70-9) |
Safety information for TRIPROLIDINE HYDROCHLORIDE
Computed Descriptors for TRIPROLIDINE HYDROCHLORIDE
TRIPROLIDINE HYDROCHLORIDE manufacturer
Malladi Drugs AND Pharmaceuticals Limited
Raj Pioneer Laboratories PVT LTD
Ralington Pharma
Rank Organics Chemical Pvt Ltd
Attar Global
Bazayan & Co.
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
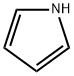
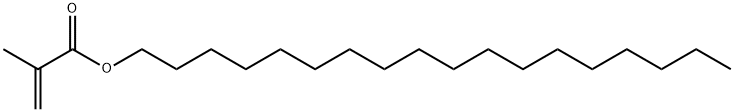
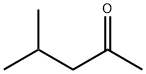
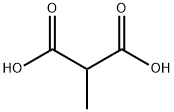
You may like
-
 550-70-9 Triprolidine hydrochloride 99%View Details
550-70-9 Triprolidine hydrochloride 99%View Details
550-70-9 -
 550-70-9 99%View Details
550-70-9 99%View Details
550-70-9 -
 Triprolidine hydrochloride 98%View Details
Triprolidine hydrochloride 98%View Details
550-70-9 -
 Triprolidine hydrochloride 550-70-9 98%View Details
Triprolidine hydrochloride 550-70-9 98%View Details
550-70-9 -
 550-70-9 98%View Details
550-70-9 98%View Details
550-70-9 -
 Triprolidine hydrochloride 97% (HPLC) CAS 550-70-9View Details
Triprolidine hydrochloride 97% (HPLC) CAS 550-70-9View Details
550-70-9 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1