Chlorotrimethylsilane
Synonym(s):TMSCl;Chlorotrimethylsilane;Trimethylsilyl chloride;TMCS;Trimethylchlorosilane
- CAS NO.:75-77-4
- Empirical Formula: C3H9ClSi
- Molecular Weight: 108.64
- MDL number: MFCD00000502
- EINECS: 200-900-5
- Update Date: 2025-09-25 17:15:13
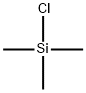
What is Chlorotrimethylsilane?
Description
Chlorotrimethylsilane is a valuable reagent for the protection of the hydroxyl function in organic synthesis. It should be distilled from calciumhydride (or tributylamine) under nitrogen before use,and stored and weighed under nitrogen. Use of the reagent without purification has been reported to lead to explosions. Chlorotrimethylsilane may contain dichlorodimethylsilane as an impurity. This may be removed before distillation by very cautious treatment with a small amount of water,which hydrolyses the dichloro compound more rapidly. Excess chlorotrimethylsilane in a reaction mixture may be destroyed by the very careful addition of aqueous sodium hydrogencarbonate solution.
Chemical properties
Trimethylchlorosilane is a colorless fuming liquid with a pungent odor. Readily hydrolyzed with liberation of hydrogen chloride; soluble in benzene, ether and perchloroethylene. Chlorotrimethylsilane is a chloro-organosilane compound mainly used for silylation reactions.
The Uses of Chlorotrimethylsilane
Chlorotrimethylsilane (TMCS) is a typical Silane Blocking Agent, which can protect or deprotect functional groups selectively. It is used in the production of trimethylsilyl halides, pseudohalides and various organic silicon compounds. It is also used to produce hexamethyldisilane by reduction. It has been used in the preparation of volatile derivatives of a wide range of compounds for GC analysis and for silylation, and as a protection group in the process of various organic syntheses. The reactions are generally performed under anhydrous conditions, although reactions can be performed on water-containing samples. There are considerable opportunities for artifacts stemming from a variety of reasons, including off-target reactions with aldehydes and ketones. The rate of reaction of TMCS with nucleophilic targets is comparatively slow compared to other alkylsilyl chlorides and requires the presence of a base catalyst such as pyridine.TMCS is included as a catalyst with another trialkylsilyl reagent such as N, O-bis- (trimethylsilyl)trifluroacetamide (BSA) or N-methyl-N-(trimethylsilyl)trifluoroacetamide (MSTFA). The order of functional group reactivity is alcohols>phenols>carboxylic acids>amines>amides.
Reactions
Related to the epoxide ring opening, TMSCl also mediates some cyclopropane ring opening reactions. For example, treatment of 1-aceto-2,2-dimethylcyclopropane with TMSCl and sodium chloride in acetonitrile at 55°C for 24 h generated 5-chloro-5-methyl-2-hexanone in 84% yield (eq 39).When sodium iodide was employed to replace sodium chloride, iodotrimethylsilane generated in situ, and the reaction completed under more facile conditions (rt and 12 h). Interestingly, the dominant product is 5-iodo-4,4-dimethyl-2-pentanone (eq 40), arising from iodide attacking at the less hindered secondary methylene carbon, instead of the quaternary dimethylmethylene carbon.
Definition
ChEBI: Chlorotrimethylsilane is a silyl chloride consisting of a central silicon atom covalently bound to one chloro and three methyl groups. Chlorotrimethylsilane is a derivatisation agent used in gas chromatography/mass spectrometry applications. It has a role as a chromatographic reagent.
General Description
Trimethylchlorosilane appears as a colorless fuming liquid with a pungent odor. Boiling point 135° F, Flash point -18°F. Density 0.854g/cm3. The vapor and liquid may cause burns. Vapors are heavier than air.
Reactivity Profile
Chlorotrimethylsilane reacts vigorously and exothermically with water to produce hydrogen chloride.
Health Hazard
Similar to other silanes. Toxicity is rated high for inhalation, ingestion and local irritation. May cause death or permanent injury after a very short exposure to small quantities.
Fire Hazard
Violent reaction with water. Toxic and irritating hydrogen chloride and phosgene may be formed in fires. Difficult to extinguish, re-ignition may occur. Flashback along vapor trail may occur. Containers may explode in fire. Vapor may explode if ignited in enclosed area. When heated to decomposition or on contact with acids or acid fumes, chloride fumes are emitted. Reacts with surface moisture, releasing hydrogen chloride, which will corrode common metals and form flammable hydrogen gas. Avoid contact with water; Chlorotrimethylsilane readily hydrolyzes, liberating hydrochloric acid. Hazardous polymerization may not occur.
Safety Profile
Poison by ingestion and skin contact. Moderately toxic by inhalation and intraperitoneal routes. A corrosive irritant to skin, eyes, and mucous membranes. Questionable carcinogen with experimental neoplastigenic data. Mutation data reported. A flammable liquid and very dangerous fire hazard when exposed to heat or flame. Violent reaction with water or hexafluoroisopropylideneamino lithium, A preparative hazard. To fight fire, use foam, alcohol foam, fog. When heated to decomposition it emits toxic fumes of Cl-. An intermediate in the production of silicones. See also CHLOROSILASES.
Potential Exposure
Trimethylchlorosilane is used as an intermediate to make silicone products, including lubricants
Shipping
UN1298 Trimethylchlorosilane, Hazard Class: 3; Labels: 3-Flammable liquid, 8-Corrosive material.
Purification Methods
Likely impurities are other chlorinated methylsilanes and tetrachlorosilane (b 57.6o), some of which can form azeotropes. To avoid the latter, very efficient fractional distillation is required. It has been fractionated through a 12 plate glass helices-packed column with only the heart-cut material being used. It has also been fractionated through a 90cm, 19mm diameter Stedman column (p 11). Purify it by redistilling from CaH2 before use. [Sauer et al. J Am Chem Soc 70, 4254 1948, Sauer & Hadsell J Am Chem Soc 70 4258 1948, Langer et al. J Org Chem 23 50 1958, Beilstein 4 IV 4007.] FLAMMABLE and CORROSIVE.
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Chlorosilanes react vigorously with bases and both organic and inorganic acids generating toxic and/or flammable gases. Chlorosilanes react with water, moist air, or steam to produce heat and toxic, corrosive fumes of hydrogen chloride. They may also produce flammable gaseous hydrogen. Attacks metals in the presence of moisture. Vigorous reaction with aluminum powder.
Waste Disposal
Do not discharge into drains or sewers. Use a licensed disposal contractor to an approved landfill. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office for guidance on acceptable disposal practices. The most favorable course of action is to use an alternative chemical product with less inherent propensity for occupational exposure or environmental contamination. Recycle any unused portion of the material for its approved use or return it to the manufacturer or supplier. Ultimate disposal of the chemical must consider: the material’s impact on air quality; potential migration in soil or water; effects on animal, aquatic, and plant life; and conformance with environmental and public health regulations.
Properties of Chlorotrimethylsilane
| Melting point: | −40 °C(lit.) |
| Boiling point: | 57 °C(lit.) |
| Density | 0.857 g/mL at 25 °C |
| vapor density | 3.7 (vs air) |
| vapor pressure | 100 mm Hg ( 25 °C) |
| refractive index | n |
| Flash point: | 104 °F |
| storage temp. | Store below +30°C. |
| solubility | Miscible with ether, benzene, diethylether and perchloroethylene. |
| form | Liquid |
| color | Clear colorless |
| Specific Gravity | 0.8536 (27℃) |
| explosive limit | 1.5-46%(V) |
| Water Solubility | REACTS |
| Sensitive | Moisture Sensitive |
| Hydrolytic Sensitivity | 8: reacts rapidly with moisture, water, protic solvents |
| BRN | 1209232 |
| Stability: | Stable. Highly flammable - note low flash point. Reacts violently with water. Incompatible with water, moisture, strong oxidizing agents, strong acids, strong bases, aldehydes, alcohols, amines, esters, ketones. |
| CAS DataBase Reference | 75-77-4(CAS DataBase Reference) |
| NIST Chemistry Reference | Silane, chlorotrimethyl-(75-77-4) |
| EPA Substance Registry System | Trimethylchlorosilane (75-77-4) |
Safety information for Chlorotrimethylsilane
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H225:Flammable liquids H312:Acute toxicity,dermal H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Chlorotrimethylsilane
| InChIKey | IJOOHPMOJXWVHK-UHFFFAOYSA-N |
Chlorotrimethylsilane manufacturer
JSK Chemicals
ASM Organics
Chemcon Speciality Chemicals Limited
Jaiswal S Cyber Shop
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 Chlorotrimethylsilane supplierView Details
Chlorotrimethylsilane supplierView Details
75-77-4 -
 Trimethyl Chlorosilane (TMCS) extrapure CAS 75-77-4View Details
Trimethyl Chlorosilane (TMCS) extrapure CAS 75-77-4View Details
75-77-4 -
 Chlorotrimethylsilane CAS 75-77-4View Details
Chlorotrimethylsilane CAS 75-77-4View Details
75-77-4 -
 Trimethyl Chloro Silane, LaboratoryView Details
Trimethyl Chloro Silane, LaboratoryView Details
75-77-4 -
 180 Kg Chloro Trimethylsilane Liquid, For Industrial, 99%View Details
180 Kg Chloro Trimethylsilane Liquid, For Industrial, 99%View Details
75-77-4 -
 170 kg Drum / Iso Tank Trimethyl Chlorosilane (TMCS), 99%View Details
170 kg Drum / Iso Tank Trimethyl Chlorosilane (TMCS), 99%View Details
75-77-4 -
 200 Kg Trimethyl Chlorosilane, 99.50%, For IndustrialView Details
200 Kg Trimethyl Chlorosilane, 99.50%, For IndustrialView Details
75-77-4 -
 JCSSUPER Chlorotrimethyl Silane For Synthesis 250 Ml., Grade Standard: Chemical GradeView Details
JCSSUPER Chlorotrimethyl Silane For Synthesis 250 Ml., Grade Standard: Chemical GradeView Details
75-77-4
