p-Tolualdehyde
Synonym(s):4-Methylbenzaldehyde;p-Tolualdehyde, p-Toluylaldehyde
- CAS NO.:104-87-0
- Empirical Formula: C8H8O
- Molecular Weight: 120.15
- MDL number: MFCD00006954
- EINECS: 203-246-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:40
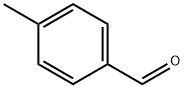
What is p-Tolualdehyde?
Chemical properties
Clear colorless to pale yellow liquid. Soluble in alcohol and oils. Sweet-herbaceous, Bitter-almond-like moderately tenacious odor. Not as pungent as Benzaldehyde, but more herbaceous-warm. Intensely sweet, warm-fruity and Bitteralmond-like taste in concentrations lower than 500 ppm.
The Uses of p-Tolualdehyde
p-Tolualdehyde is made by the Vilsmeier reaction employing toluene and dimethylformamide or the Guttermann–Koch reaction employing toluene and carbon monoxide.
The Uses of p-Tolualdehyde
p-Tolualdehyde is used as an intermediate for the synthesis of pharmaceuticals, dyes perfumes and agrochemicals. It is also used as a fixative of flavorings. It is also used as an important organic intermediates, used for spices, triphenylmethane dye synthesis, etc.
The Uses of p-Tolualdehyde
p-Tolualdehyde may be used as an analytical reference standard for the quantification of the analyte in the following:
- Air samples using high-performance liquid chromatography with UV detection (HPLC-UV).
- Mango cultivars using gas chromatography (GC) and gas chromatography-mass spectrometry (GC-MS).
What are the applications of Application
p-Tolualdehyde is a pharmaceutical intermediate
Definition
ChEBI: A tolualdehyde compound with the methyl substituent at the 4-position.
Preparation
p-Tolualdehyde is prepared by oxidation of mixed Tolylalcohols.
Synthesis Reference(s)
Journal of the American Chemical Society, 105, p. 7175, 1983 DOI: 10.1021/ja00362a028
Tetrahedron Letters, 29, p. 6265, 1988 DOI: 10.1016/S0040-4039(00)82321-7
General Description
p-Tolualdehyde (4-Methylbenzaldehyde) is an aromatic aldehyde. It has been generated as major oxygenated product during the UV light irradiated oxygenation of p-xylene, via photoinduced electron transfer mechanism. It undergoes condensation reaction with diiron μ-ethylidyne complex to afford μ-vinylcarbyne complex (92% yield).
Flammability and Explosibility
Not classified
Purification Methods
Steam distil the aldehyde, dry it with CaSO4, then fractionally distil it. [Beilstein 7 IV 672.]
Properties of p-Tolualdehyde
| Melting point: | -6 °C |
| Boiling point: | 204-205 °C (lit.)
82-85 °C/11 mmHg (lit.) |
| Density | 1.019 g/mL at 25 °C (lit.) |
| vapor pressure | 0.33 hPa (25 °C) |
| FEMA | 3068 | TOLUALDEHYDES (MIXED O,M,P) |
| refractive index | n |
| Flash point: | 176 °F |
| storage temp. | Store below +30°C. |
| solubility | water: soluble0.25 g/L at 25°C |
| form | Liquid |
| color | Clear colorless to yellow |
| Odor | at 5.00 % in dipropylene glycol. fruity cherry deep phenolic |
| explosive limit | 0.9-5.6%(V) |
| Water Solubility | 0.25 g/L (25 ºC) |
| Sensitive | Air Sensitive |
| BRN | 385772 |
| CAS DataBase Reference | 104-87-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Benzaldehyde, 4-methyl-(104-87-0) |
| EPA Substance Registry System | 4-Methylbenzaldehyde (104-87-0) |
Safety information for p-Tolualdehyde
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for p-Tolualdehyde
| InChIKey | FXLOVSHXALFLKQ-UHFFFAOYSA-N |
p-Tolualdehyde manufacturer
BTC pharm India
JSK Chemicals
ASM Organics
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 104-87-0 p-Tolualdehyde, 99% 99%View Details
104-87-0 p-Tolualdehyde, 99% 99%View Details
104-87-0 -
 4-Methyl benzaldehyde CAS 104-87-0View Details
4-Methyl benzaldehyde CAS 104-87-0View Details
104-87-0 -
 p-Tolualdehyde CAS 104-87-0View Details
p-Tolualdehyde CAS 104-87-0View Details
104-87-0 -
 P-Tolualdehyde 95% CAS 104-87-0View Details
P-Tolualdehyde 95% CAS 104-87-0View Details
104-87-0 -
 4-Methylbenzaldehyde CAS 104-87-0View Details
4-Methylbenzaldehyde CAS 104-87-0View Details
104-87-0 -
 Liquid Industrial Grade 4-Methylbenzaldehyde (104-87-0) (C8H8O), 50 litre Drum, 95-97%View Details
Liquid Industrial Grade 4-Methylbenzaldehyde (104-87-0) (C8H8O), 50 litre Drum, 95-97%View Details
104-87-0 -
 Liquid 99% Min 4-Methylbenzaldehyde, Packaging Type: DrumView Details
Liquid 99% Min 4-Methylbenzaldehyde, Packaging Type: DrumView Details
104-87-0 -
 p-Tolualdehyde 104-87-0 98+View Details
p-Tolualdehyde 104-87-0 98+View Details
104-87-0
