Pyrrole
Synonym(s):Azole;Divinylenimine;Imidole;Pyrrole
- CAS NO.:109-97-7
- Empirical Formula: C4H5N
- Molecular Weight: 67.09
- MDL number: MFCD00005216
- EINECS: 203-724-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
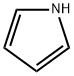
What is Pyrrole?
Physical properties
Pyrrole is a colorless to brown liquid that has a sweet, warm-ethereal smell, similar to chloroform. It dissolves in ethanol, ether, benzene, dilute acids, and most non-volatile oils but does not dissolve in water or dilute alkalis. When stored for extended periods, it tends to aggregate and become brown due to the influence of light.
Chemical properties
Six π-electrons are distributed over the five ring atoms of pyrrole. Delocalization of these electrons stabilizes the ring and the lone pair of electrons on the nitrogen atom, which is responsible for the usual basicity of nitrogen compounds, is involved in the electron cloud, and is not available for sharing. Hence, pyrrole is an extremely weak base and the pyrrolic nitrogen is not readily susceptible to electrophilic enzymic attack (Damani, 1985). There is a high electron density, however, at all positions of the ring, which causes pyrrole to be reactive toward electrophilic substitution. In general, electrophilic substitution reactions on the neutral molecule occur preferentially at the C-2 or C-5 positions (Jones and Bean, 1977; Damani and Crooks, 1982).
Occurrence
The pyrrole ring is the basic unit of the porphyrin system which occurs, for
example, in chlorophyll and in hemoglobin. Other pyrrole-based natural products
include pigments such as bilirubin and biliverdin, which are degradative products
from porphyrins (Sundberg, 1984).
Pyrrole has been found in surface waters and in filtrates from cultures of the
blue-green algae, Anabaenaflos aquae. The presence of pyrrole and other organic
nitrogen compounds in natural waters is of environmental concern because they
may exert significant chlorine demand. Pyrrole is also a precursor to trihalomethane
formation (Ram and Morris, 1980).
At ambient temperature, pyrrole can be volatilized from shale oil wastewaters.
Concentrations of approximately 3 g/m3 have been measured indoors in air at an
oil shale wastewater facility (Hawthorne and Sievers, 1984). Pyrrole has been
identified in tobacco smoke, although not in tobacco itself (Johnstone and Plim-mer, 1959); in cigarette smoke (Schumacher et al 1977); in cigar butt aroma (Peck
et al 1969); and in Cannabis smoke condensate (Jones and Foote, 1975).
Pyrrole was found to be naturally occurring in foods; in fact, it is on the Food
and Drug Administration GRAS (Generally Recognized As Safe) list, with an
average usage level of 3 p.p.m. in flavoring formulations (Maga, 1981). Pyrrole is
a volatile constituent of roasted coffee (Gianturco et al 1966), roasted peanuts
(Walradt et al 1971), and fried chicken (Tang et al 1983). It has also been
identified in beef aroma (MacLeod and Coppock, 1976) and is a constituent of
cocoa aroma (Marion et al 1967). It should be noted that all the foods listed have
undergone some degree of thermal treatment; pyrrole was not present in the fresh,
raw foods. In model system studies, pyrrole was among the resulting compounds
when hydroxyproline and glucose were heated under nitrogen at temperatures
ranging from 120° to 200°C. Large amounts of pyrrole were found, as well, when
casein and collagen were pyrolyzed and when proline underwent high temperature
pyrolysis (Maga, 1981).
The Uses of Pyrrole
Commercial applications of this compoundare very limited. It is used in organic synthesis.Pyrrole is formed by heating albumin orby pyrolysis of gelatin.
The Uses of Pyrrole
Pyrrole plays a major role in synthesis of drugs, spices, agrochemicals, dyes, photographic chemicals and perfumes. It plays an important role in the electropolymerisation of macroporous conducting polymer films. It acts as a catalyst for polymerization process; as a standard substance in chromatographic analysis; as corrosion inhibitors and preservatives and as solvents for resins and terpenes. It is utilized to study the hydrogen-bond mediated coupling of 1,2,3-triazole to pyrrole and in the preparation of 1-(4-Chloro-benzoyl)-pyrrole by reacting with 4-Chloro-benzoyl chloride. In Ciamician-Dennstedt rearrangement, It is used to prepare 3-chloropyridine by reacting with dichlorocarbene.
What are the applications of Application
Pyrrole is a simple organic molecule that is useful in organic synthesis and many other applications
Definition
ChEBI: 1H-pyrrole is a tautomer of pyrrole that has the double bonds at positions 2 and 4. It is a pyrrole and a secondary amine. It is a tautomer of a 2H-pyrrole and a 3H-pyrrole.
Production Methods
Pyrrole originally was prepared industrially by fractional distillation of coal tar, bone oil or other protein material, and purified through formation of its potassium derivative (Runge, 1834; Michelman, 1925). Later it was produced by heating ammonium mucate with glycerol or mineral oil (Blicke and Powers, 1927; McElvain and Bollinger, 1941). It is now manufactured by addition of ammonia to either acetylene or butadiene. Good yields of pyrrole also may be obtained from the reaction of ammonia with the corresponding heterocyclic compound (furan) in a vapor-phase process at 480° to 500°C, using alumina as a catalyst (Thompson, 1972) or by catalytic reaction of furan with ammonia over a molybdenum or vanadium oxide catalyst at 350-400°C (Bishop and Denton, 1950).
Preparation
By fractional distillation of bone oil (bone oil is obtained by destructive distillation of animal bone) and subsequent purification via the corresponding potassium salt; by thermal decomposition of ammonium mucate in glycerol or mineral oil.
Production Methods
Pyrrole may be made (1) by reaction of succinimide with zinc and acetic acid, or with hydrogen in the presence of finely divided platinum heated, (2) by reaction of ammonium saccharate or mucate COONH4·(CHOH)4·COONH4 with glycerol at 200 °C by loss of carbon dioxide, ammonia, and water. When pyrrole is treated with potassium (but not with sodium) or boiled with solid potassium hydroxide, potassium pyrrole C4H4NK is formed, which is the starting point for N-derivatives of pyrrole, since reaction of the potassium with halogen of organic compound and with carbon dioxide, readily occurs.
Aroma threshold values
Detection: 20 to 49.6 ppm
General Description
Pyrrole is one of the flavor compounds that is formed in thermally processed foods due to the Maillard reaction.
Hazard
Moderate fire risk. Toxic by ingestion and inhalation.
Health Hazard
Pyrrole is harmful if swallowed, inhaled, or absorbed through the skin. Its vapor or mist is irritating to the eyes, mucous membranes and upper respiratory tract (Lenga, 1985; Sax, 1984). Although no cases of occupational disease due to pyrrole have been reported, it has a depressant action on the central nervous system and, in severe intoxication, it is injurious to the liver. Tests indicate that it has moderate cumulative toxicity (Parmegianni, 1983).
Health Hazard
The toxicity data on pyrrole are scant. Itis moderately toxic on test animals. Theroutes of exposure are inhalation of vapors,ingestion, and skin absorption. Vapors arean irritant to the eyes and respiratory tract.The lethal doses in rabbits by oral anddermal routes are within the range 150 and250 mg/kg, respectively.
Fire Hazard
Combustible liquid; flash point (closed cup) 39°C (102°F); vapor forms explosive mixtures with air; LEL and UEL values are not available. Heating with strong oxidizers can be violent.
Industrial uses
Pyrrole is used to a limited extent as a solvent for polymeric esters, but its primary value lies in its function as a chemical intermediate. It is used in the synthesis of non-heterocyclic compounds (Kozikowski, 1984) and its derivatives have been used in the manufacture of dyes, herbicides, perfumes, and as cross-linking agents for curing resins (Thompson, 1972). Derivatives of pyrrole are utilized in pharmaceutical applications, particularly as anti-inflammation drugs and drugs with central nervous system activity, including antihypertensive effects (Sundberg, 1984); and as antimicrobial agents (Freeman, 1975), such as fungicides (Zirngibl, 1983) and bactericides (Bailey and Johnson, 1973; Bailey et al 1973; Sundberg, 1984). Polymers of pyrrole have been used in the preparation of photoconductive materials. The main utility of poly(pyrrole) has been for the modification of electrode surfaces, although numerous other applications can be envisioned (Heilmann and Rasmussen, 1984).
Industrial uses
Pyrrole is a five-member nitrogen heterocyclic ring that contains two carbon-carbon
double bond configurations which gives the solvent a pronounced aromatic
character. Pyrrole is an intermediate in the synthesis of a variety of commercial
chemical derivatives. Pyrrole has only limited solubility
in water but are miscible with many organic solvents.Pyrrole when freshly distilled
is a colorless liquid, but the solvent can rapidly acquire a brown coloration due to
air oxidation. Prolonged standing in the air will promote slow polymerization of the
pyrrole to give a dark brown polymer. Pyrrole has a viscosity of 1.31 centipoise
and a medium surface tension value of 37.1 dynes/cm.
pyrrole is used as a chemical intermediate in the
preparation of electrically conducting polypyrrole by means of an electrochemical
polymerization process. Pyrrole has few other industrial uses.
Safety Profile
Poison by ingestion, subcutaneous, and intraperitoneal routes. Flammable liquid when exposed to heat or flame; can react with oxilzing materials. To fight fire, use foam, CO2, dry chemical. Violent reaction with 2-nitrobenzaldehyde. When heated to decomposition it emits highly toxic fumes of NOx.
Metabolism
Reports concerning the metabolites formed following administration of pyrrole
have been somewhat confusing. Saccardi (1919a, 1920) observed that administration
of pyrrole orally and by injection resulted in the formation of melanin in the
urine of rabbits, but not of dogs. Unchanged pyrrole was also found in the urine of
rabbits after injection of pyrrole (Saccardi, 1919b). Shimizu (1921) isolated
methylpyridine from the urine of rabbits and dogs given pyrrole and suggested that
pyrrole could be converted to pyridine derivatives in vivo. The transformations in
the body and the excretion products in the urine are, however, in question
(Fairhall, 1969). Novello (1927) injected rabbits subcutaneously with 0.5 g doses
of pyrrole hydrochloride and attempted to detect acetyl or methyl derivatives, but
was unsucessful. Approximately 40-50% of the nitrogen of the injected pyrrole
was excreted as urea. By the process of elimination, Novello (1927) concluded that the nitrogen not accounted for as urea nitrogen was excreted as unchanged
pyrrole. It did not appear that the pyrrole was oxidized to a secondary or tertiary
alcohol because there was no rise in ethereal sulfate or conjugated glucuronic acid
excretion. Kusui (1935) injected frogs with pyrrole and noted that although the
urine smelled of pyrrole, no free base could be isolated. Damani and Crooks
(1982) have suggested that pyrrole may be a likely substrate for hydroxylation at
C-2 and C-5, leading to ring opened products. They have not, however, studied the
biotransformation of pyrrole, but based their hypothesis on studies of the metabolism
of indole.
Pyrrole may affect the biotransformation of other compounds. Bernheim et al
(1938) observed that pyrrole acted as a catalyst for the oxidation of amines and
certain non-natural amino acids and catalyzed the formation of methemoglobin
from hemoglobin. On the other hand, pretreatment of rats with 100 mg/kg pyrrole
inhibited markedly the metabolism of dimethylnitrosamine in terms of both C02
excretion and decline in blood dimethylnitrosamine concentration (Phillips et al
1982).
Purification Methods
Dry pyrrole with NaOH, CaH2 or CaSO4. Fractionally distil it under reduced pressure from CaH2. Store it under nitrogen as it turns brown in air. Redistil it immediately before use. The picrate forms orange-red crystals with m 69o(dec). [Beilstein 20 H 4, 20 I 3, 20 II 3, 20 III/IV 61, 20/5 V 3.]
Properties of Pyrrole
| Melting point: | -23 °C (lit.) |
| Boiling point: | 131 °C (lit.) |
| Density | 0.967 g/mL at 25 °C (lit.) |
| vapor density | 2.31 (vs air) |
| vapor pressure | 8.7 hPa (20 °C) |
| FEMA | 3386 | PYRROLE |
| refractive index | n |
| Flash point: | 92 °F |
| storage temp. | Store at +2°C to +8°C. |
| solubility | 60g/l |
| form | Liquid |
| pka | 15(at 25℃) |
| color | Clear almost colorless to brownish |
| PH | >6 (10g/l, H2O, 20℃) |
| Odor | at 0.10 % in propylene glycol. sweet warm nutty ethereal |
| explosive limit | 3.10-14.8%(V) |
| Water Solubility | 60 g/L (20 ºC) |
| Sensitive | Air & Light Sensitive |
| Merck | 14,8014 |
| JECFA Number | 1314 |
| BRN | 1159 |
| Dielectric constant | 7.5(17℃) |
| Stability: | Stable. Incompatible with strong acids, strong oxidizing agents. Combustible. |
| CAS DataBase Reference | 109-97-7(CAS DataBase Reference) |
| NIST Chemistry Reference | Pyrrole(109-97-7) |
| EPA Substance Registry System | 1H-Pyrrole (109-97-7) |
Safety information for Pyrrole
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H226:Flammable liquids H301:Acute toxicity,oral H318:Serious eye damage/eye irritation H332:Acute toxicity,inhalation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Pyrrole
| InChIKey | KAESVJOAVNADME-UHFFFAOYSA-N |
Pyrrole manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
![1,2,3,4-TETRAHYDRO-9H-PYRIDO[3,4-B]INDOLE](https://img.chemicalbook.in/CAS/GIF/16502-01-5.gif)
![pyrrolo[1,2-a]quinoxaline](https://img.chemicalbook.in/CAS/GIF/234-95-7.gif)
You may like
-
 109-97-7 Pyrrole, 98% 99%View Details
109-97-7 Pyrrole, 98% 99%View Details
109-97-7 -
 Pyrrole extrapure CAS 109-97-7View Details
Pyrrole extrapure CAS 109-97-7View Details
109-97-7 -
 Pyrrole CASView Details
Pyrrole CASView Details -
 Pyrrole CAS 109-97-7View Details
Pyrrole CAS 109-97-7View Details
109-97-7 -
 Pyrrole CAS 109-97-7View Details
Pyrrole CAS 109-97-7View Details
109-97-7 -
 Pyrrole CAS 109-97-7View Details
Pyrrole CAS 109-97-7View Details
109-97-7 -
 PYRROLE (109-97-7), LiquidView Details
PYRROLE (109-97-7), LiquidView Details
109-97-7 -
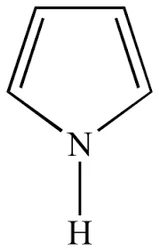 PyrroleView Details
PyrroleView Details
109-97-7
