2-Methylindole
Synonym(s):NSC 7514
- CAS NO.:95-20-5
- Empirical Formula: C9H9N
- Molecular Weight: 131.17
- MDL number: MFCD00005616
- EINECS: 202-398-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-04-09 17:45:08
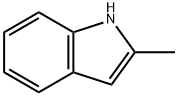
What is 2-Methylindole?
Chemical properties
Colorless needle or yellow to reddish-purple or brown crystals, flakes. Has an animal type odor.Soluble in ethanol and ether, insoluble in water.
The Uses of 2-Methylindole
2-Methylindole is an intermediate in the synthesis of indole derivative with potential antifungal activities. It can be used as a raw material for the preparation of deacetylase (HDAC) inhibitor panobinostat.
The Uses of 2-Methylindole
2-Methylindole is used as a reactant for regioselective synthesis of oxopyrrolidine analogs via iodine-catalyzed Markovnikov addition reaction, Friedel-Crafts alkylation reactions, preparation of tryptophan dioxygenase inhibitors pyridyl-ethenyl-indoles as potential anticancer immunomodulators, Michael addition reactions and in synthesis of cyclooxygenase-1 (COX-1)/cyclooxygenase-2 (COX-2) inhibitors.
What are the applications of Application
2-Methylindole is a reactant for Friedel-Crafts alkylation reactions
Definition
ChEBI: 2-Methylindole is a methylindole that is 1H-indole substituted by a methyl group at position 2. It derives from a hydride of a 1H-indole.
What are the applications of Application
2-Methylindole is used as a reactant Reactant for:
Regioselective synthesis of oxopyrrolidine analogs via iodine-catalyzed Markovnikov addition reaction
Friedel-Crafts alkylation reactions
Preparation of tryptophan dioxygenase inhibitors pyridyl-ethenyl-indoles as potential anticancer immunomodulators
Preparation of plant-growth inhibitors
Michael addition reactions
Synthesis of cyclooxygenase-1 (COX-1)/cyclooxygenase-2 (COX-2) inhibitors
Preparation
2-Methylindole was synthesized from 2-Acetamidotoluene by the following procedure. 2-Acetamidotoluene was added to the mixture of anhydrous ether and sodium amide, heated to 240-260°C under the protection of nitrogen flow, kept for 10min, a large amount of gas was generated in the reaction, and the reaction ended when the gas stopped escaping, and cooled. Ethanol and warm water were added and heated to decompose the sodium derivative of 2-Methylindole and excess sodium amide. After cooling, it was extracted with ether. The extract was concentrated and then distilled, and the fractions at 119-126°C (0.4-0.53kPa) were collected to obtain 2-Methylindole with a yield of 80%-83%. The product can be purified by methanol recrystallization.
Synthesis Reference(s)
Journal of the American Chemical Society, 98, p. 2674, 1976 DOI: 10.1021/ja00425a051
Organic Syntheses, Coll. Vol. 3, p. 597, 1955
Tetrahedron Letters, 9, p. 3499, 1968
Purification Methods
Crystallise it from *benzene. It has also been purified by zone melting. The picrate has m 139o (from Et2O or Et2O/MeOH). [Cohen et al. J Am Chem Soc 82 2184 1960, Beilstein 20 III/IV 3202, 20/7 V 59.]
Properties of 2-Methylindole
| Melting point: | 57-59 °C (lit.) |
| Boiling point: | 273 °C (lit.) |
| Density | 1,07 g/cm3 |
| refractive index | 1.6070 (estimate) |
| Flash point: | 141 °C |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| solubility | Chloroform (Sparingly), Methanol (Slightly) |
| pka | 17.57±0.30(Predicted) |
| form | Crystals, Flakes, or Crystalline Powder |
| color | Yellow to reddish-purple or brown |
| Odor | at 0.10 % in dipropylene glycol. animal indole fresher than skatole with no fecal odor |
| Water Solubility | Insoluble in water. |
| Sensitive | Light Sensitive |
| BRN | 109781 |
| CAS DataBase Reference | 95-20-5(CAS DataBase Reference) |
| NIST Chemistry Reference | 1H-Indole, 2-methyl-(95-20-5) |
| EPA Substance Registry System | 1H-Indole, 2-methyl- (95-20-5) |
Safety information for 2-Methylindole
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P501:Dispose of contents/container to..… |
Computed Descriptors for 2-Methylindole
| InChIKey | BHNHHSOHWZKFOX-UHFFFAOYSA-N |
2-Methylindole manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 95-20-5 2 Methyl Indole 99%View Details
95-20-5 2 Methyl Indole 99%View Details
95-20-5 -
 2-Methylindole 98%View Details
2-Methylindole 98%View Details -
 95-20-5 99%View Details
95-20-5 99%View Details
95-20-5 -
 2-Methylindole CAS 95-20-5View Details
2-Methylindole CAS 95-20-5View Details
95-20-5 -
 2-Methyl indole CAS 95-20-5View Details
2-Methyl indole CAS 95-20-5View Details
95-20-5 -
 2-Methylindole CAS 95-20-5View Details
2-Methylindole CAS 95-20-5View Details
95-20-5 -
 2 Methyl Indole, Grade Standard: IndustrialView Details
2 Methyl Indole, Grade Standard: IndustrialView Details
95-20-5 -
 2 MethylIndole 95-20-5View Details
2 MethylIndole 95-20-5View Details
95-20-5
