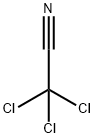
Trichloroacetyl chloride
- CAS NO.:76-02-8
- Empirical Formula: C2Cl4O
- Molecular Weight: 181.83
- MDL number: MFCD00000792
- EINECS: 200-926-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
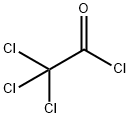
What is Trichloroacetyl chloride?
Chemical properties
Trichloroacetyl chloride is similar to dichloroacetyl chloride. It is a clear colourless liquid. Decomposes in water; soluble in alcohol.The acid chloride can be hydrolyzed at 75 – 85°C with water to form the free acid and hydrolyzed with ammonium hydroxide or concentrated sodium carbonate solution to form the salts.
The Uses of Trichloroacetyl chloride
Trichloroacetyl chloride can be used for the manufacture of the esters and the anhydrides of trichloroacetic acid.It was used in the preparation of dihydro-1H-benzindoles. It was also used in the synthesis of 3-alkylbenzoxazolones.
The Uses of Trichloroacetyl chloride
Trichloroacetyl Chloride is a reagent used to synthesize pyrazol-furan carboxamide analogues, which can act as Akt kinase inhibitors and pyrrolostatin, which can act as a lipid peroxidation inihibitor (1,2).
Preparation
The acetyl chloride is prepared from trichloroacetic acid and various inorganic acid chlorides (e.g., SOCl2, PCl3) or with P2O5 and HCl. More useful methods are the oxidation of tetrachloroethylene with fuming sulfuric acid, oxygen, or fuming nitric acid and sulfuric acid at 18-20°C, or from the reaction of pentachloroethane and dry oxygen under UV light. It has been obtained in 37 % yield from carbon tetrachloride and carbon monoxide in the presence of aluminum chloride at 200°C and high pressure.The most common production method is the gas-phase, photochemical oxidation of tetrachloroethylene with oxygen. The reaction is initiated with UV light, with radioactive irradiation, or it is sensitized with chlorine or iodine.
General Description
A colorless volatile liquid with a strong odor. Denser than water. Contact severely irritates skin, eyes and mucous membranes. May be very toxic by ingestion and inhalation. May be combustible.
Reactivity Profile
Trichloroacetyl chloride is incompatible with water, with strong oxidizing agents, alcohols, bases (including amines). May react vigorously or explosively if mixed with diisopropyl ether or other ethers in the presence of trace amounts of metal salts [J. Haz. Mat., 1981, 4, 291].
Health Hazard
Highly toxic by ingestion and inhalation; strong irritant to skin and tissues.
Fire Hazard
Material may burn but does not ignite readily. Poisonous if inhaled or swallowed; skin contact poisonous. Contact may cause burns to skin and eyes.
Potential Exposure
Used in chemical syntheses.
Shipping
UN2442 Trichloroacetyl chloride, Hazard class: 8; Labels: 8-Corrosive material, 6.1-Poisonous materials.
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, ethers and metal salts. Violent reaction with water, forming hydrochloric acid and trichloroacetic acid.
Waste Disposal
Do not discharge into drains or sewers. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. Controlled incineration (oxides of nitrogen are removed from the effluent gas by scrubbers and/or thermal devices) Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office.
Properties of Trichloroacetyl chloride
| Melting point: | -57 °C |
| Boiling point: | 114-116 °C(lit.) |
| Density | 1.629 g/mL at 25 °C(lit.) |
| vapor pressure | 16 mm Hg ( 20 °C) |
| refractive index | n |
| Flash point: | 100 °C |
| storage temp. | Refrigerator, Under inert atmosphere |
| solubility | Chloroform (Soluble), Ethyl Acetate (Soluble) |
| form | Oil |
| color | Colourless |
| Water Solubility | reacts violently |
| Sensitive | Moisture Sensitive |
| BRN | 774120 |
| Stability: | Stable. Reacts violently with water. Incompatible with alcohols, oxidizing agents, strong bases. |
| CAS DataBase Reference | 76-02-8(CAS DataBase Reference) |
| NIST Chemistry Reference | Trichloroacetyl chloride(76-02-8) |
| EPA Substance Registry System | Trichloroacetyl chloride (76-02-8) |
Safety information for Trichloroacetyl chloride
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H314:Skin corrosion/irritation H318:Serious eye damage/eye irritation H330:Acute toxicity,inhalation |
| Precautionary Statement Codes |
P320:Specific treatment is urgent (see … on this label). P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for Trichloroacetyl chloride
Trichloroacetyl chloride manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

You may like
-
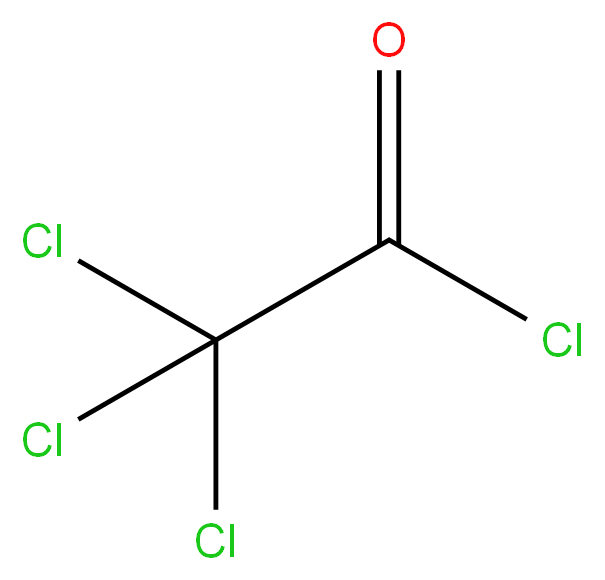 Trichloro Acetyl Chloride 76-02-8 99%View Details
Trichloro Acetyl Chloride 76-02-8 99%View Details
76-02-8 -
 76-02-8 Trichloroacetyl chloride 98%View Details
76-02-8 Trichloroacetyl chloride 98%View Details
76-02-8 -
 76-02-8 98%View Details
76-02-8 98%View Details
76-02-8 -
 Trichloroacetyl chloride 76-02-8 98%View Details
Trichloroacetyl chloride 76-02-8 98%View Details
76-02-8 -
 Trichloro Acetyl Chloride (TCAC) 0.99View Details
Trichloro Acetyl Chloride (TCAC) 0.99View Details
76-02-8 -
 Trichloroacetyl chloride 95% CAS 76-02-8View Details
Trichloroacetyl chloride 95% CAS 76-02-8View Details
76-02-8 -
 Trichloroacetyl chloride, 98% CAS 76-02-8View Details
Trichloroacetyl chloride, 98% CAS 76-02-8View Details
76-02-8 -
 Trichloroacetyl chloride CAS 76-02-8View Details
Trichloroacetyl chloride CAS 76-02-8View Details
76-02-8
