Trichloroacetic acid
Synonym(s):TCA;Trichloroacetic acid;Trichloroacetic Acid - CAS 76-03-9 - Calbiochem
- CAS NO.:76-03-9
- Empirical Formula: C2HCl3O2
- Molecular Weight: 163.39
- MDL number: MFCD00004177
- EINECS: 200-927-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
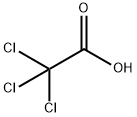
What is Trichloroacetic acid?
Description
Trichloroacetic acid, also known as TCA or 76-03-9, is a colorless or white orthorhombic crystal with strong deliquescence and a slight, special irritant smell. It is highly corrosive. TCA's aqueous solution is strongly acidic, with a pH of 1.2 for a 0.1 mol solution. Concentrations of TCA at or below 30% cannot be stored for long periods due to decomposition into chloroform, hydrogen chloride, carbon dioxide, and carbon monoxide. Dilute alkali leads to hydrolysis into chloroform and carbon dioxide. Concentrated alkali results in the formation of formic acid. TCA is a highly toxic substance with an oral LD50 of 3320mg/kg.
Chemical properties
Trichloroacetic acid, a colorless crystalline solid, is commonly utilized in liquid solutions. It has the ability to absorb moisture from the surrounding air and become syrupy. When dissolved in water, the process releases heat. However, this potent acid is corrosive to both metals and tissue.
The Uses of Trichloroacetic acid
Protein precipitation reagentTrichloroacetic acid is used as a precipitating agent in biochemistry for precipitation of proteins, DNA and RNA. It is an active ingredient used in cosmetic treatments like chemical peels, tattoo removal and the treatment of warts including genital warts. It is also used to determine protein concentration and as a decalcifier and fixative in microscopy.
The Uses of Trichloroacetic acid
Traditionally used to precipitate protein. Has been used to determine protein concentration by quantitative precipitation. Used as decalcifier and fixative, as a laboratory reagent, a herbicide, in medicine, and in microscopy.
The Uses of Trichloroacetic acid
Trichloroacetate (TCA) is used primarily for the selective control of annual and perennial grass weeds in cropland and noncropland. TCA is acidic in nature and are not strongly sorbed by soils. It is reported to be rapidly degraded in both soil and water by microbial processes. However, the breakdown of TCA occurs very slowly when incubated at 14–15 °C in acidic soils. iming not only accelerates this degradation but also increases the numbers of TCA-degrading bacteria.
The Uses of Trichloroacetic acid
As a reagent for albumin detection; in making herbicides. It is found as a by-product after chlorination of water containing humic materials.
Background
Trichloroacetate, or trichloroacetic acid, is a strong acid prepared by the reaction of chlorine with acetic acid in the presence of a suitable catalyst. In clinical chemistry and biochemistry, it is used as a precipitant of macromolecules including proteins, DNA and RNA. Trichloroacetate is also found in cosmetic treatments and topical formulations as a cauterant, which removes condyloma or warts on the skin.
What are the applications of Application
Trichloroacetic acid is an acetic acid analogue commonly used to precipitate proteins, DNA and RNA
Definition
ChEBI: Trichloroacetic acid is a monocarboxylic acid that is acetic acid in which all three methyl hydrogens are substituted by chlorine. It has a role as a metabolite, a carcinogenic agent and a mouse metabolite. It is a monocarboxylic acid and an organochlorine compound. It is functionally related to an acetic acid. It is a conjugate acid of a trichloroacetate.
What are the applications of Application
Trichloroacetic acid(76-03-9) can be used as pharmaceutical raw materials, herbicides (potassium trichloroacetate and sodium trichloroacetate, etc.), textile dyeing auxiliaries, metal surface treatment agent and acid chloride, anhydride, amide, polyester, organometallic salt, water salicylaldehyde, chlorocarboxylic acid and the raw materials of other organic synthesis.
In addition, in medicine, it can also be used as etherifying agents and keratolytics, bile pigment reagents and protein precipitation reagents. In the field of biochemistry, it can be used for separation analysis of biological phosphate compounds and reagents for determination of fluoride and lipid as well as microscopic fixative, decalcification, chromatography reagents.
The product is warts agent and astringent in pharmaceutical field, mainly used as biochemical drug extractant for the extraction of many highly efficient drugs such as adenosine triphosphate, cytochrome C and placental polysaccharides.
In addition, trichloroacetic acid, together with alkaline phenol, can be used for salicylaldehyde compound synthesis by ReimerTiemann reaction. It can also react with monoolefine compounds for synthesizing chlorocarboxylic acid [CCl3 (CH2CH2) nCOOH].
Preparation
Trichloroacetic acid is produced on an industrial scale by chlorination of acetic acid or chloroacetic acid mother-liquors at 140 - 160 °C. If necessary, calcium hypochlorite is added as a chlorination accelerator. There are conflicting views concerning adding heavy metal salts as chlorination catalysts. Examples of catalysts that have been used are iron and copper compounds, which are precipitated with sulfuric acid or phosphoric acid if decomposition of the reaction mixture occurs; 2% phosphoric acid; and catalysts and UV light. Trichloroacetic acid has also been produced without catalysts. The crude product, containing about 95% trichloroacetic acid, is best isolated by crystallizing the melt, removing the mother-liquor with most of its impurities, and increasing the purity by centrifugation or recrystallization.
Reactions
Trichloroacetic acid is a strong organic acid with a dissociation constant K = 3 × 10-2. It has lively chemical properties. Its sodium salt is easily subject to decarboxylation into chloroform. It will be reduced to alcohol upon coming across LiAlH4. It can have halogen replacement reaction with KBr:
General Description
Trichloroacetic acid (TCA) is derived from Trichloroethylene (TCE) metabolism. It is used as an acid decalcifying agent. TCA is used as a fixative for nuclear staining and protein precipitation.
Reactivity Profile
Trichloroacetic acid is a strong acid; when heated, in the presence of water, decomposes forming phosgene and HCl. [Handling Chemicals Safely 1980 p. 915]. The acid was added to copper wool and rinsed down with dimethyl sulfoxide. This caused what was thought to be an extremely exothermic dehydrohalogenation reaction that melted the neck of the flask, [Chem. Eng. News, 1981, 59(28), 4].
Health Hazard
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Combustible material: may burn but does not ignite readily. When heated, vapors may form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated. Runoff may pollute waterways. Substance may be transported in a molten form.
Biochem/physiol Actions
TCA might act as a carcinogen, targeting liver. It might be used as an acid decalcifying agent. TCA helps in nuclear staining.
Safety Profile
Poison by ingestion and subcutaneous routes. Moderately toxic by intraperitoneal route. Questionable carcinogen with experimental carcinogenic data. Experimental reproductive effects. Mutation data reported. A corrosive irritant to skin, eyes, and mucous membranes. When heated to decomposition it emits toxic fumes of Cland Na2O. Used as an herbicide.
Potential Exposure
This haloacetic acid can be a byproduct of drinking water disinfection and may increase the risk of cancer. Trichloroacetic acid is used as medication; in organic syntheses; as a reagent for albumin detection; as an intermediate in pesticide manufacture and in the production of sodium trichloroacetate which is itself a herbicide.
Carcinogenicity
TCA was not mutagenic in bacterial assays.The IARC has determined that there is limited evidence for the carcinogenicity of TCA in experimental animals and that it is not classifiable as to its carcinogenicity to humans. Neutralized TCA was not clastogenic in human lymphocytes in vitro or in the mouse bone marrow micronucleus test.
Metabolism
Not Available
Shipping
UN1839 (solid) & UN2564 (solution) Trichloroacetic acid, solid and Trichloroacetic acid, solution, Hazard class: 8; Labels: 8-Corrosive material.
Purification Methods
Purify the acid by fractional crystallisation from its melt, then crystallise it repeatedly from dry *benzene and store it over conc H2SO4 in a vacuum desiccator. It can also be crystallised from CHCl3 or cyclohexane, and dried over P2O5 or Mg(ClO4)2 in a vacuum desiccator. Trichloroacetic acid can be fractionally distilled under reduced pressure from MgSO4. Layne, Jaffé and Zimmer [J Am Chem Soc 85 435 1963] dried trichloroacetic acid in *benzene by distilling off the *benzene-water azeotrope, then crystallised the acid from the remaining *benzene solution. Manipulations should be carried out under N2. [Toxic vapours, use a well ventilated fume cupboard.] [Beilstein 2 IV 508.]
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, silver salts, strong acids, strong bases, moisture, iron, zinc, aluminum. Corrosive to iron, steel and other metals.
References
https://pubchem.ncbi.nlm.nih.gov/compound/trichloroacetic_acid#section=Top
https://en.wikipedia.org/wiki/Trichloroacetic_acid
Properties of Trichloroacetic acid
| Melting point: | 54-58 °C (lit.) |
| Boiling point: | 196 °C (lit.) |
| Density | 1.62 g/mL at 25 °C (lit.) |
| vapor density | <1 (vs air) |
| vapor pressure | 1 mm Hg ( 51 °C) |
| refractive index | n |
| Flash point: | 196°C |
| storage temp. | Store at +15°C to +25°C. |
| solubility | H2O: 0.5 M at 20 °C, clear, colorless |
| form | Solid |
| pka | 0.7(at 25℃) |
| color | White |
| PH | <1.0 (25℃, 0.5M in H2O) |
| Odor | sharp, pungent odor |
| Water Solubility | 120 g/100 mL (20 ºC) |
| Sensitive | Hygroscopic |
| λmax | 210nm(EtOH)(lit.) |
| Merck | 14,9627 |
| BRN | 970119 |
| Dielectric constant | 4.9(20℃) |
| Exposure limits | ACGIH: TWA 0.5 ppm NIOSH: TWA 1 ppm(7 mg/m3) |
| Stability: | Stable, but moisture sensitive. Incompatible with water, strong bases. Note that the Merck Index states that this material is hydrolytically unstable in aqueous solution below 30% by weight. Decomposition products include carbon monoxide and carbon dioxide. The generation of these gases in a sealed container may lead to a pressure rise sufficien |
| CAS DataBase Reference | 76-03-9(CAS DataBase Reference) |
| NIST Chemistry Reference | Acetic acid, trichloro-(76-03-9) |
| IARC | 2B (Vol. 63, 84, 106) 2014 |
| EPA Substance Registry System | Trichloroacetic acid (76-03-9) |
Safety information for Trichloroacetic acid
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Trichloroacetic acid
Trichloroacetic acid manufacturer
JSK Chemicals
ASM Organics
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 Trichloroacetic acid, For ACS analysis CAS 76-03-9View Details
Trichloroacetic acid, For ACS analysis CAS 76-03-9View Details
76-03-9 -
 Trichloroacetic acid, For ACS analysis CAS 76-03-9View Details
Trichloroacetic acid, For ACS analysis CAS 76-03-9View Details
76-03-9 -
 TRICHLOROACETIC ACID 99%View Details
TRICHLOROACETIC ACID 99%View Details -
 Trichloroacetic Acid 20% solution CAS 76-03-9View Details
Trichloroacetic Acid 20% solution CAS 76-03-9View Details
76-03-9 -
 Trichloroacetic Acid extrapure AR CAS 76-03-9View Details
Trichloroacetic Acid extrapure AR CAS 76-03-9View Details
76-03-9 -
 Trichloroacetic Acid extrapure CAS 76-03-9View Details
Trichloroacetic Acid extrapure CAS 76-03-9View Details
76-03-9 -
 Trichloroacetic acid 99.00% CAS 76-03-9View Details
Trichloroacetic acid 99.00% CAS 76-03-9View Details
76-03-9 -
 Tri Chloro Acetic Acid 10, Powder, Packaging Size: 25 KgsView Details
Tri Chloro Acetic Acid 10, Powder, Packaging Size: 25 KgsView Details
76-03-9
