Trichloroacetonitrile
Synonym(s):Trichloroacetonitrile;Trichloromethylnitrile
- CAS NO.:545-06-2
- Empirical Formula: C2Cl3N
- Molecular Weight: 144.39
- MDL number: MFCD00001842
- EINECS: 208-885-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
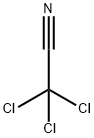
What is Trichloroacetonitrile?
Chemical properties
colourless to slightly yellow liquid
The Uses of Trichloroacetonitrile
Trichloroacetonitrile is involved as a reagent in Overman rearrangement, which is used to prepare alylic amines from allylic alcohols. It is also used to prepare bistrichloroacetimidates from diols leading to dihyrooxazines through acid catalyzed cyclization. Further, it is utilized in the synthesis of trichloroacetimidates by 1,8-Diazobicyclo[5.4.0]undec-7-ene (DBU) catalyzed addition of allylic alcohols. It finds application in the study of the methoxy methyl (MOM) catalyzed aza-Claisen rearrangement.
The Uses of Trichloroacetonitrile
Insecticide.
What are the applications of Application
Trichloroacetonitrile is Used for the synthesis of trichloroacetimidates and bistrichloroacetimidates
Definition
ChEBI: Trichloroacetonitrile is an aliphatic nitrile and an organochlorine compound.
Production Methods
Trichloroacetonitrile can be obtained by dehydration of trichloroacetamide with phosphorous pentoxide or by chlorination of acetonitrile with chlorine. Vapor phase chlorination in the presence of water and photochemical chlorination in the presence of catalysts such as HgCl2 or AlCl3 have been reported. Trichloroacetonitrile is an organic intermediate used, for example, in the synthesis of the fungicide etridiazole.
Air & Water Reactions
Highly flammable. Insoluble in water.
Reactivity Profile
May be sensitive to light and heat. Trichloroacetonitrile may react with water, steam, acid or acid fumes. Trichloroacetonitrile may hydrolyze under acidic or alkaline conditions. . The reaction of benzene and Trichloroacetonitrile evolves toxic chloroform and HCl gasses. (Hagedorn, F., H.-P. Gelbke, and Federal Republic of Germany. 2002. Nitriles. In Ullman Encyclopedia of Industrial Chemistry. Wiley-VCH Verlag GmbH & Co. KGaA.).
Hazard
Strong irritant to tissue. Questionable carcinogen.
Fire Hazard
Trichloroacetonitrile is combustible.
Safety Profile
Poison by ingestion and intravenous routes. Moderately toxic by inhalation and skin contact. Human mutation data reported. A skin and severe eye irritant. An experimental teratogen. Other experimental reproductive effects. When heated to decomposition or in reaction with water, steam, acid, or acid fumes it produces toxic fumes of CN-, Cl-, and NOx. Used as an insecticide. See also NITRILES and CYANIDE.
Properties of Trichloroacetonitrile
| Melting point: | -42 °C |
| Boiling point: | 83-84 °C(lit.) |
| Density | 1.44 g/mL at 25 °C(lit.) |
| vapor pressure | 58 mm Hg ( 20 °C) |
| refractive index | n |
| Flash point: | None |
| storage temp. | Store below +30°C. |
| solubility | <0.1 g/100 mL at 21.5°C |
| form | Liquid |
| color | Clear colorless to very slightly yellow |
| Odor | odor of chloral and hydrogen cyanide |
| Water Solubility | <0.1 g/100 mL at 21.5 ºC |
| Sensitive | Lachrymatory |
| Merck | 14,9628 |
| BRN | 605572 |
| Exposure limits | NIOSH: IDLH 25 mg/m3 |
| Dielectric constant | 4.6(60℃) |
| Stability: | Stable, but water sensitive. Incompatible with acids, water, steam. May hydrolyze in alkali or acid conditions. Flammable. |
| CAS DataBase Reference | 545-06-2(CAS DataBase Reference) |
| IARC | 3 (Vol. 52, 71) 1999 |
| NIST Chemistry Reference | Acetonitrile, trichloro-(545-06-2) |
| EPA Substance Registry System | Trichloroacetonitrile (545-06-2) |
Safety information for Trichloroacetonitrile
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for Trichloroacetonitrile
Trichloroacetonitrile manufacturer
JSK Chemicals
ASM Organics
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 Trichloroacetonitrile 98%View Details
Trichloroacetonitrile 98%View Details -
 Trichloroacetonitrile 99%View Details
Trichloroacetonitrile 99%View Details
545-06-2 -
 Trichloroacetonitrile, 97% 99%View Details
Trichloroacetonitrile, 97% 99%View Details
545-06-2 -
 Trichloroacetonitrile CAS 545-06-2View Details
Trichloroacetonitrile CAS 545-06-2View Details
545-06-2 -
 Trichloroacetonitrile 97% CAS 545-06-2View Details
Trichloroacetonitrile 97% CAS 545-06-2View Details
545-06-2 -
 Trichloroacetonitrile CAS 545-06-2View Details
Trichloroacetonitrile CAS 545-06-2View Details
545-06-2 -
 Tri Chloro AcetonitrileView Details
Tri Chloro AcetonitrileView Details
545-06-2 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0
