CERIUM(III) CHLORIDE
Synonym(s):Cerium trichloride;Cerous chloride
- CAS NO.:7790-86-5
- Empirical Formula: CeCl3
- Molecular Weight: 246.48
- MDL number: MFCD00010929
- EINECS: 232-227-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
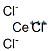
What is CERIUM(III) CHLORIDE?
Chemical properties
Cerium(III) chloride is a white powder and is used for incandescent gas mantles, spectrography and polymerization of olefins.
Cerium(III) chloride is mainly used as catalyst for petroleum,automobile exhaust catalyst , also used as a raw material for the production of cerium metal and other cerium compounds.
Physical properties
White, very fine powder; hexagonal crystal system; heptahydrate is yellow orthogonal crystal and hygroscopic; density of anhydrous salt 3.97 g/cm3; melts at 817°C; vaporizes at 1,727°C; heptahydrate begins to lose water above 90°C and becomes anhydrous at about 230°C; soluble in water and alcohol; hexahydrate has greater solubility in these solvents.
The Uses of CERIUM(III) CHLORIDE
Cerium(III) chloride is used in incandescent gas mantles, spectrography and polymerization of olefins. It acts as a Lewis acid and used in Friedel-Crafts alkylation and acylation reactions in organic synthesis. It is used as a precursor for the preparation of other cerium salts, such as cerium(III) trifluoromethanesulfonate.
Production Methods
Cerium(III) chloride is prepared by the reaction of hydrochloric acid with a cerium salt, such as cerium hydroxide or carbonate, followed by crystallization;
Ce(OH)3 + 3HCl → CeCl3 + 3H2O
Ce2(CO3)3 + 6HCl → 2CeCl3 + 3CO2 + 3H2O.
Flammability and Explosibility
Non flammable
Safety Profile
Poison by intravenous, intraperitoneal, and subcutaneous routes. Moderately toxic by ingestion. See also CERIUM COMPOUNDS. When heated to decomposition it emits toxic fumes of Cl-
Properties of CERIUM(III) CHLORIDE
| Melting point: | 848 °C(lit.) |
| Boiling point: | 1727 °C |
| Density | 3.97 g/mL at 25 °C(lit.) |
| Flash point: | 1727°C |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | soluble in H2O, ethanol |
| form | beads |
| color | White to Pale Gray |
| Specific Gravity | 3.92 |
| Water Solubility | Soluble in water, ethanol, acids and acetone. |
| Sensitive | Hygroscopic |
| Merck | 14,1997 |
| Stability: | Stable. Hygroscopic. Incompatible with strong acids, strong oxidizing agents. |
| CAS DataBase Reference | 7790-86-5(CAS DataBase Reference) |
| EPA Substance Registry System | Cerium chloride (CeCl3) (7790-86-5) |
Safety information for CERIUM(III) CHLORIDE
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Environment GHS09 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for CERIUM(III) CHLORIDE
| InChIKey | VYLVYHXQOHJDJL-UHFFFAOYSA-K |
CERIUM(III) CHLORIDE manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 Cerium(III) chloride, ultra dry, (REO) CAS 7790-86-5View Details
Cerium(III) chloride, ultra dry, (REO) CAS 7790-86-5View Details
7790-86-5 -
 Cerium(III) chloride CAS 7790-86-5View Details
Cerium(III) chloride CAS 7790-86-5View Details
7790-86-5 -
 Cerium(III) chloride CAS 7790-86-5View Details
Cerium(III) chloride CAS 7790-86-5View Details
7790-86-5 -
 Cerium(III) chloride CAS 7790-86-5View Details
Cerium(III) chloride CAS 7790-86-5View Details
7790-86-5 -
 Cerium(III) chloride CAS 7790-86-5View Details
Cerium(III) chloride CAS 7790-86-5View Details
7790-86-5 -
 Cerium(III) chloride CAS 7790-86-5View Details
Cerium(III) chloride CAS 7790-86-5View Details
7790-86-5 -
 Cerium(III) chloride CAS 7790-86-5View Details
Cerium(III) chloride CAS 7790-86-5View Details
7790-86-5 -
 CERIUM CHLORIDE ANHYDROUS cas no 7790-86-5, For IndustrialView Details
CERIUM CHLORIDE ANHYDROUS cas no 7790-86-5, For IndustrialView Details
7790-86-5
