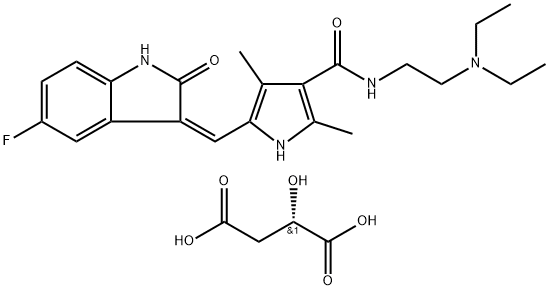
Sunitinib Malate
Synonym(s):N-[2-(Diethylamino)ethyl]-5-[(Z)-(5-fluoro-1,2-dihydro-2-oxo-3H-indol-3-ylidene)methyl]-2,4-dimethyl-1H-pyrrole-3-carboxamide (2S)-2-hydroxybutanedioic acid (1:1) salt;SU 011248;SU 11248;SU112248
- CAS NO.:341031-54-7
- Empirical Formula: C26H33FN4O7
- Molecular Weight: 532.57
- MDL number: MFCD08282795
- EINECS: 638-825-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 21:30:31
What is Sunitinib Malate?
Description
Sunitinib is a small molecule inhibitor of receptor tyrosine kinases, including FLK1 (Ki = 9 nM), PDGFRβ (Ki = 8 nM), and FLT3. It is at least 10-fold selective for FLK1 and PDGFRβ over a variety of tyrosine kinases in a panel, including EGFR, Cdk2, Met, IGFR-1, Abl, and Src. Sunitinib inhibits VEGF-dependent FLK1 and PDGF-dependent PDGFRβ phosphorylation (IC50s = 10 and 10 nM, respectively) as well as phosphorylation of FLT3 and FLT3 carrying the activating internal tandem duplication mutation (FLT3-ITD; IC50s = 250 and 50 nM, respectively). It decreases VEGF- and FGF-induced proliferation of human umbilical vein endothelial cells (HUVECs; IC50s = 30 and 700 nM, respectively) and reduces tumor growth in a variety of mouse xenograft models when administered at doses ranging from 20 to 80 mg/kg per day. Formulations containing sunitinib have been used in the treatment of gastrointestinal stromal tumors and metastatic renal cell carcinoma.
Chemical properties
Yellow Solid
The Uses of Sunitinib Malate
Sunitinib Malate is a multi-targeted RTK inhibitor targeting VEGFR2 (Flk-1) and PDGFRβ with IC50 of 80 nM and 2 nM, and also inhibits c-Kit
The Uses of Sunitinib Malate
Sunitinib Malate (Sutent, SU11248) is a multi-targeted RTK inhibitor targeting VEGFR2 (Flk-1) and PDGFRβ with IC50 of 80 nM and 2 nM, and also inhibits c-Kit.
The Uses of Sunitinib Malate
A multi-kinase inhibitor targeting several receptor tyrosine kinases (RTK). Antineoplastic.
What are the applications of Application
Sunitinib Malate is an antineoplastic agent, Flk-1 inhibitor, and ATP-competitve inhibitor of PDGFRβ
brand name
(Pfizer).
General Description
Sunitinib is available in 12.5-, 25-, and 50-mg capsules fororal administration for the treatment of advanced RCC andGIST upon the failure of imatinib. The agent is a multikinaseinhibitor and has been shown to inhibit PDGF-R,VEGF-R, Kit, RET, and the colony-stimulating factor receptor(CSR-1R). The result of this spectrum of activity isa slowing of tumor progression and inhibition of angiogenesisboth of which are useful in the highly vascularizedcancers, RCC and GIST. The median TTP (time to tumorprogression) was 27.3 weeks for sunitinib and 6.4 weeks with a median time of 24.1 weeks for sunitinib and 6 weeksfor placebo. The agent is well absorbed upon oral administration,and both the parent and major metabolite are highly(90%–95%) protein bound. Metabolism is mediated primarilyby CYP3A4 to give the N-desethyl derivative, which isthe major metabolite (23%–37%), equally active with theparent and undergoes further metabolism by CYP3A4. Theterminal elimination half-life for the parent and N-desethylderivative are 40 to 60 hours and 80 to 110 hours, respectively.Elimination occurs primarily via the feces. Commonadverse effects of sunitinib include fatigue, diarrhea, yellowskin discoloration, anorexia, nausea, and mucositis.Mild myelosuppression has been reported in patients withGIST including neutropenia, lymphopenia, thrombocytopenia,and anemia. There have been reports of cardiotoxicityincluding decreases in left ventricular ejectionfraction, which occurred in 11% of patients during a GISTstudy.
Biological Activity
Potent, ATP-competitive VEGFR, PDGFR β and KIT inhibitor (K i values are 2, 9, 17, 8 and 4 nM for VEGFR -1, -2, -3, PDGFR β and KIT respectively). Also inhibits cellular receptor phosphorylation of FLT3, RET and CSF-1R. Exhibits antiangiogenic and antitumor activity in multiple xenograft models.
Biochem/physiol Actions
Sunitinib has greater bioavailability and potency compared to other inhibitors. It prevents angiogenesis.
Storage
Store at +4°C
References
1) Deeks et al. (2006), Sunitinib; Drugs, 66 2255 2) Guo et al. (2006), Inhibition of phosphorylation of the colony-stimulating factor-1 receptor (c-Fms) tyrosine kinase in transfected cells by ABT-869 and other tyrosine kinase inhibitors; Mol. Cancer. Ther., 5 1007 3) O’Farrell et al. (2003), SU11248 is a novel FLT3 yrosine kinase with potent activity in vitro and in vivo; Blood, 101 3597 4) Roskoski et al. (2007), Sunitinib: a EGF and PDGF receptor protein kinase and angiogenesis inhibitor; Biochem. Biophys. Res. Commun., 356 323
Properties of Sunitinib Malate
| Melting point: | 189-191°C |
| storage temp. | room temp |
| solubility | DMSO: >10mg/mL |
| form | powder |
| color | Orange |
| Stability: | Stable for 2 years from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 3 months. |
| CAS DataBase Reference | 341031-54-7(CAS DataBase Reference) |
Safety information for Sunitinib Malate
| Signal word | Danger |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H360:Reproductive toxicity H372:Specific target organ toxicity, repeated exposure |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Sunitinib Malate
| InChIKey | XGQXULJHBWKUJY-LYIKAWCPSA-N |
Sunitinib Malate manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 341031-54-7 99%View Details
341031-54-7 99%View Details
341031-54-7 -
 341031-54-7 Sunitinib malate 98%View Details
341031-54-7 Sunitinib malate 98%View Details
341031-54-7 -
 341031-54-7 98%View Details
341031-54-7 98%View Details
341031-54-7 -
 341031-54-7 Sunitinib malate 98%View Details
341031-54-7 Sunitinib malate 98%View Details
341031-54-7 -
 341031-54-7 SUNITINIB 95-99%View Details
341031-54-7 SUNITINIB 95-99%View Details
341031-54-7 -
 Sunitinib malate 99% (HPLC) CAS 341031-54-7View Details
Sunitinib malate 99% (HPLC) CAS 341031-54-7View Details
341031-54-7 -
 Sunitinib malate 95% CAS 341031-54-7View Details
Sunitinib malate 95% CAS 341031-54-7View Details
341031-54-7 -
 Sunitinib malate CAS 341031-54-7View Details
Sunitinib malate CAS 341031-54-7View Details
341031-54-7
