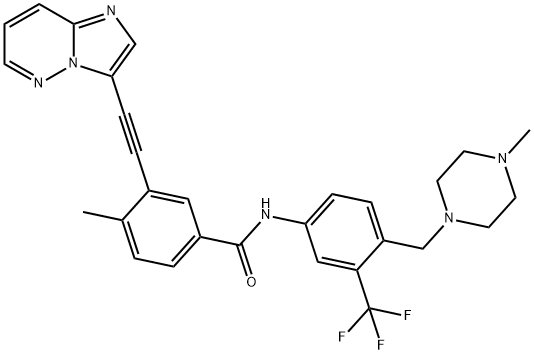
Ponatinib
- CAS NO.:943319-70-8
- Empirical Formula: C29H27F3N6O
- Molecular Weight: 532.56
- MDL number: MFCD17215203
- EINECS: 1308068-626-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 15:53:33
What is Ponatinib?
Absorption
The absolute bioavailability of ponatinib is unknown. Peak concentrations of ponatinib are observed within 6 hours after Iclusig oral administration. Food does not affect absorption of food. The aqueous solubility of ponatinib is pH dependent, with higher pH resulting in lower solubility. When 45 mg of ponatinib is given to cancer patients, the pharmacokinetic parameters are as follows: Cmax = 73 ng/mL; AUC = 1253 ng?hr/mL;
Toxicity
The most common non-hematologic adverse reactions (≥ 20%) were hypertension, rash, abdominal pain, fatigue, headache, dry skin, constipation, arthralgia, nausea, and pyrexia. Hematologic adverse reactions included thrombocytopenia, anemia, neutropenia, lymphopenia, and leukopenia.
Description
Ponatinib (943319-70-8) is a highly potent pan-Bcr-Abl and multikinase inhibitor, suppressing the activity of native Bcr-Abl (IC50=0.37 nM) as well as the mutants T315I (IC50=2 nM), Q252H (IC50=0.44 nM), Y253F (IC50=0.3 nM), M351T (IC50=0.3 nM) and H396P (IC50=0.34 nM).1??Also inhibits PDGFRα, c-Src and c-Kit (IC50=1.1, 5.4 and 12.5 nM respectively)2?as well as FGFR-mediated signaling (IC50<40 nM)3. Protects against influenza A virus-induced death by suppressing cytokine storm in mouse models..4
Chemical properties
Pale Yellow Solid
The Uses of Ponatinib
Ponatinib (AP24534) is a novel potent, orally available small molecule multitargeted kinase inhibitor. Ponatinib inhibits both native and mutant BCR-ABL. Ponatinib is used in the treatment of chronic myeloid leukemia (CML) with BCR-ABL kinase inhibitors.
The Uses of Ponatinib
AP24534 is a novel potent, orally available small molecule multitargeted kinase inhibitor with IC50 of 0.37, 2, 1.5, 2.2, 1.1, 1and 0.24 nM for native pan-BCR-ABL, mutated form, VEGFR2, FGFR1, PDGFRα, mutant FLT3 phosphorylation and LYN.
The Uses of Ponatinib
Ponatinib (AP24534) is a novel, potent multi-target inhibitor of Abl, PDGFRα, VEGFR2, FGFR1 and Src with IC50 of 0.37 nM, 1.1 nM, 1.5 nM, 2.2 nM and 5.4 nM, respectively
Indications
Ponatinib is indicated for the treatment of adult patients with chronic phase, accelerated phase, or blast phase chronic myeloid leukemia (CML) that is resistant or intolerant to prior tyrosine kinase inhibitor therapy or Philadelphia chromosome positive acute lymphoblastic leukemia (Ph+ALL) that is resistant or intolerant to prior tyrosine kinase inhibitor therapy.
Background
Ponatinib is a novel Bcr-Abl tyrosine kinase inhibitor that is especially effective against the T315I mutation for the treatment of chronic myeloid leukemia. FDA approved on December 14, 2012.
What are the applications of Application
AP 24534 is a Bcr-Abl (Bcr and c-Abl fusion), FGFR, and Flk-1 inhibitor
Definition
ChEBI: A benzamide obtained by the formal condensation of the carboxy group of 3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methylbenzoic acid with the anilino group of 4-[(4-methylpiperazin-1-yl)methyl]-3-(trifluoromethyl)aniline.
brand name
IclusigTM
General Description
Ponatinib, a third-generation BCR-ABL tyrosine kinase inhibitor (TKI), was first approved in December 2012 as a third-line treatment for CML patients. However, three months later, its approval was suspended, primarily due to safety concerns arising from the increased incidence of serious vascular occlusive events. Because there was no alternative treatment option at the time for CML patients with the T315I mutation, the suspension was lifted after a couple of months, and ponatinib was reintroduced for adult patients with T315I-postive CML (including accelerated, chronic, or blast phases) or those with T315I-positive Ph+ ALL with multiple safety measures regarding cardiovascular risk.
Class: non-receptor tyrosine kinase
Treatment: CML
Elimination half-life = 24 h
Protein binding = 99%
Clinical Use
Protein kinase inhibitor:
Treatment of chronic myeloid leukaemia (CML)
Treatment of Philadelphia chromosome positive
acute lymphoblastic leukaemia (Ph+ ALL)
Drug interactions
Potentially hazardous interactions with other drugs
Antibacterials: concentration reduced by rifampicin
- avoid.
Antipsychotics: avoid concomitant use with
clozapine (increased risk of agranulocytosis).
Metabolism
At least 64% of a ponatinib dose undergoes phase I and phase II metabolism. CYP3A4 and to a lesser extent CYP2C8, CYP2D6 and CYP3A5 are involved in the phase I metabolism of ponatinib in vitro. Ponatinib is also metabolized by esterases and/or amidases.
Metabolism
Ponatinib is metabolised to an inactive carboxylic acid by
esterases and/or amidases, and metabolised by CYP3A4
to an N-desmethyl metabolite that is 4 times less active
than ponatinib.
Ponatinib is mainly eliminated via faeces. Following a
single oral dose of [14C]-labelled ponatinib, approximately
87% of the radioactive dose is recovered in the faeces and
approximately 5% in the urine.
storage
Store at -20°C
References
1) O’Hare?et al.?(2009),?AP24534, a pan-BCR-ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation-based resistance; Cancer Cell,?16?401 2) Gozgit?et al.?(2011),?Potent activity of ponatinib (AP24535) in models of FLT3-driven acute myeloid leukemia and other hematologic malignancies; Mol. Cancer Ther.,?10?1028 3) Gozgit?et al.?(2012),?Ponatinib (AP24534), a multitargeted pan-FGFR inhibitor with activity in multiple FGFR-amplified or mutated cancer models; Mol. Cancer Ther.,?11?690 4) Chen?et al.?(2019),?Ponatinib Protects Mice From Lethal Influenza Infection by Suppressing Cytokine Storm; Front. Immunol., 10 1393
Properties of Ponatinib
| Melting point: | >175°C (dec.) |
| Density | 1.3 |
| storage temp. | -20°C |
| solubility | Soluble in DMSO (up to 50 mg/ml) or in Ethanol (up to 25 mg/ml with warming). |
| form | Yellow powder. |
| pka | 12.99±0.70(Predicted) |
| color | Yellow |
| Stability: | Stable for 2 years from date? of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20° for up to 3 months. |
| CAS DataBase Reference | 943319-70-8 |
Safety information for Ponatinib
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H372:Specific target organ toxicity, repeated exposure |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for Ponatinib
Ponatinib manufacturer
New Products
Trans-methyl 4-aminocyclohexane- carboxylate HCl 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-Pyridineacetonitrile, α-hydroxy- 3-Iodophenylacetic acid (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine Cyclohexane, (2-propynyloxy)- 3-Bromobenzaldehyde, 95% 2-Naphthol, 98% Cysteamine hydrochloride, 98% Copper(II) bromide, 98% 1-Chloro-2,4-difluorobenzene,98% Dodecylbenzenesulfonic acid, 95% L-Glycine methyl ester.HCl Fmoc-L-Tyr(tBu)-OH Calcium Alphaketoglutarate* H-Ser(t-Bu)-Ser(t-Bu)-Gly-OH Fmoc-Ser(tBu)-Ser(Ψ(Me,Me)pro-OH Triphosgene 5-Cyanophthalide 10-Methoxy-5H-dibenz[b,f]azepine L-Glutamic Acid Dimethyl Ester Hcl 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base)Related products of tetrahydrofuran
You may like
-
 Ponatinib 98% (HPLC) CAS 943319-70-8View Details
Ponatinib 98% (HPLC) CAS 943319-70-8View Details
943319-70-8 -
 15761-38-3 N-Boc-L-Alanine >98%View Details
15761-38-3 N-Boc-L-Alanine >98%View Details
15761-38-3 -
 6485-34-3 Saccharin Calcium >98%View Details
6485-34-3 Saccharin Calcium >98%View Details
6485-34-3 -
 873-74-5 4-Aminobenzonitrile 99%View Details
873-74-5 4-Aminobenzonitrile 99%View Details
873-74-5 -
 872-85-5 99%View Details
872-85-5 99%View Details
872-85-5 -
 N,N-Carbonyl diimidazole 99%View Details
N,N-Carbonyl diimidazole 99%View Details
530-62-1 -
 3- Pyridinecarboxaldehdye 500-22-1 99%View Details
3- Pyridinecarboxaldehdye 500-22-1 99%View Details
500-22-1 -
 80841-78-7 DMDO-Chloride 92%View Details
80841-78-7 DMDO-Chloride 92%View Details
80841-78-7