Sorafenib tosylate
- CAS NO.:475207-59-1
- Empirical Formula: C21H16ClF3N4O3.C7H8O3S
- Molecular Weight: 637.03
- MDL number: MFCD08235032
- EINECS: 641-758-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 21:30:31
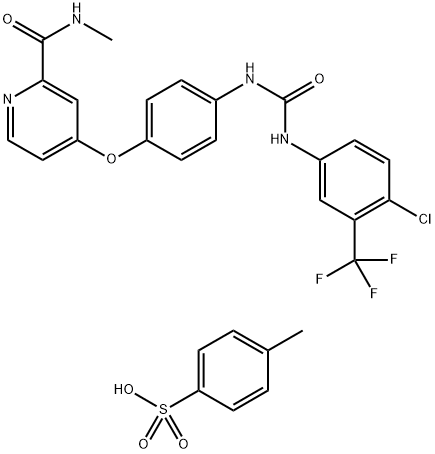
What is Sorafenib tosylate?
Description
Sorafenib (475207-59-1) was initially developed as a Raf kinase inhibitor, IC50 = 6 nM, but has been shown to inhibit many receptor tyrosine kinases including BRAF (IC50 = 22 nM); VEGFR-2 (IC50 = 90 nM); VEGFR-3 (IC50 = 20 nM); PDGFR-β (IC50 = 57 nM); Flt3 (IC50 = 58 nM); c-KIT (IC50 = 68 nM); FGFR-1 (IC50 = 580 nM).1?Paradoxically more potent in a cellular assay (IC50 = 20 nM) compared to an isolated enzyme assay (IC50 = 107 nM) for c-Fms.2?Inhibits activation of MAPK pathway and ERK phosphorylation.3?Induces caspase-independent apoptosis in melanoma cells.4?Sorafenib is a clinically useful anticancer agent.
The Uses of Sorafenib tosylate
Sorafenib Tosylate (Bay 43-9006, Nexavar) is a small molecular inhibitor of VEGFR, PDGFR, c-Raf and B-Raf with IC50s of 18 nM, 10 nM, 3 nM and 15 nM, respectively.
What are the applications of Application
Sorafenib Tosylate is an inhibitor of Flk-1 (VEGFR), PDGFR and Raf kinases used for the treatment of human hepatocellular carcinoma.
Definition
ChEBI: Sorafenib tosylate is an organosulfonate salt. It contains a sorafenib.
brand name
Nexavar (Bayer).
General Description
Sorafenib is available in 200-mg tablets for oral administrationand is used in the treatment of RCC and colon cancer.The agent is classified as a multikinase inhibitor because ofits action on numerous kinase enzymes including thePDGF-R, VEGF-R, Kit, and Raf. Sorafenib is 39% to 48% bioavailable and CYP3A4-mediated metabolism giveseight identified metabolites including the N-oxide, which isequally active with the parent. However, the majority of thedrug in plasma is present as the parent compound.Sorafenib is highly protein bound (99.5%). The drug iseliminated primarily in the feces (77%) with 19% appearingin the urine as glucuronides (UGT1A1) and has a eliminationhalf-life of 24 to 48 hours. The most commonly seentoxicity is skin rash that normally occurs within the first6 weeks of therapy. Other adverse effects include hypertension,fatigue, increased wound healing time, and increasedrisk of bleeding.
Clinical Use
Sorafenib is used to treat late-stage kidney cancer (advanced renal cell carcinoma), liver cancer (hepatocellular carcinoma) that cannot be treated by surgery, and differentiated thyroid cancer that has come back or spread to other parts of your body. Sorafenib is an antineoplastic (cancer) agent. It interferes with the growth of cancer cells, which are eventually destroyed by the body.
Side Effects
The side effects of Sorafenib tosylate include Bleeding gums, blistering, peeling, redness, or swelling of the palms of the hands or bottoms of the feet, bloating of the abdomen or stomach, blood in the urine or stools, clay-colored stools, coughing up blood, difficulty with breathing or swallowing, increased menstrual flow or vaginal bleeding.
References
1) Wilhelm et al. (2004), BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis; Cancer Res., 64 7099 2) Guo et al. (2006), Inhibition of phosphorylation of the colony-stimulating factor-1 receptor (c-Fms) tyrosine kinase in transfected cells by ABT-869 and other tyrosine kinase inhibitors; Mol. Cancer Ther., 5 1007 3) Wilhelm et al. (2003), The novel Raf inhibitor BAY 43-9006 blocks signaling and proliferation in BRAF mutant and wildtype melanoma and colorectal tumor cell lies; Proc. Am. Assoc. Cancer Res., 44 106609 4) Panka et al. (2006), The Raf inhibitor BAY 43-9006 (Sorafenib) induces caspase-independent apoptosis in melanoma cells; Cancer Res., 66 1611
Properties of Sorafenib tosylate
| Melting point: | 229-232°C |
| storage temp. | 2-8°C(protect from light) |
| solubility | Soluble in DMSO (up to 200 mg/ml) or in Ethanol (up to 3 mg/ml). |
| form | solid |
| color | Off-white |
| Stability: | Stable for 2 years from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20° for up to 1 month. |
| CAS DataBase Reference | 475207-59-1(CAS DataBase Reference) |
Safety information for Sorafenib tosylate
| Signal word | Danger |
| Pictogram(s) |
 Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H362:Reproductive toxicity, effects on or via lactation H372:Specific target organ toxicity, repeated exposure H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P263:Avoid contact during pregnancy/while nursing. P273:Avoid release to the environment. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Sorafenib tosylate
| InChIKey | IVDHYUQIDRJSTI-UHFFFAOYSA-N |
| SMILES | S(C1C=CC(C)=CC=1)(O)(=O)=O.O(C1C=CC(NC(=O)NC2C=CC(Cl)=C(C(F)(F)F)C=2)=CC=1)C1=CC=NC(C(=O)NC)=C1 |
Sorafenib tosylate manufacturer
Sumar biotech LLP
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran



You may like
-
 475207-59-1 98%View Details
475207-59-1 98%View Details
475207-59-1 -
 Sorafenib Tosylate 99%View Details
Sorafenib Tosylate 99%View Details -
 Sorafenib tosylate 98% (HPLC) CAS 475207-59-1View Details
Sorafenib tosylate 98% (HPLC) CAS 475207-59-1View Details
475207-59-1 -
 Sorafenib tosylate 98% CAS 475207-59-1View Details
Sorafenib tosylate 98% CAS 475207-59-1View Details
475207-59-1 -
 Sorafenib tosilate CAS 475207-59-1View Details
Sorafenib tosilate CAS 475207-59-1View Details
475207-59-1 -
 Sorafenib for peak identification CAS 475207-59-1View Details
Sorafenib for peak identification CAS 475207-59-1View Details
475207-59-1 -
 Sorafenib Tosylate CAS 284461-73-0View Details
Sorafenib Tosylate CAS 284461-73-0View Details
475207-59-1 -
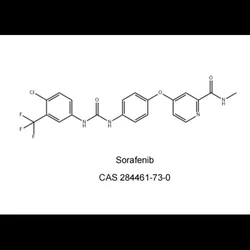 Sorafenib Tosylate Cas 284461 73 0, Packaging Size: 30 Tablets, 200 mgView Details
Sorafenib Tosylate Cas 284461 73 0, Packaging Size: 30 Tablets, 200 mgView Details
475207-59-1
