Vemakalant
- CAS NO.:794466-70-9
- Empirical Formula: C20H31NO4
- Molecular Weight: 349.46
- MDL number: MFCD16038313
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-28 23:16:16
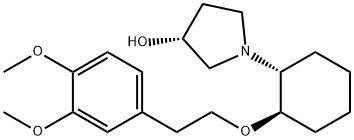
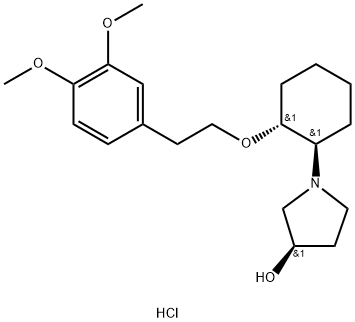
What is Vemakalant?
Absorption
In patients, average peak plasma concentrations of vernakalant were 3.9 μg/ml following a single 10 minute infusion of 3 mg/kg vernakalant hydrochloride, and 4.3 μg/ml following a second infusion of 2 mg/kg with a 15 minute interval between doses .
Toxicity
Some common unwanted effects include hypotension, ventricular arrhythmias, bradycardia, atrial flutter, dysgeusia, paraesthesia, dizziness and nausea.
Description
Vernakalant is a potassium channel blocker that was approved in Europe in 2010 for treatment of atrial fibrillation (AF), a condition of cardiac arrhythmia in which the atria of the heart beat irregularly due to changes in cardiac ion channel function and distribution. Vernakalant has activity for cardiac Na+ and K+ channels and also for the atrial-selective Kv1.5 channel. Vernakalant (RSD1235) was characterized in animal models to assess efficacy, atrial selectivity, and reduction in side effects.
Originator
Cardiome Pharma Corp. (Canada)
Indications
Indicated for the rapid conversion of recent onset of atrial fibrillation to sinus rhythm in adults for non-surgery patients that lasts for less than 7 days of duration and post-cardiac surgery patients with atrial fibrillation lasting less than 3 days of duration.
Background
Vernakalant was developed by Cardiome Pharma as as an antiarrhythmic drug intended for rapid conversion of atrial fibrillation to sinus rhythm. It acts as an atypical class III antiarrhythmic drug that potentiates its effect in higher heart rates. Intravenous formulation was approved in Europe in September 2010 as Brinavess and in Canada in April 2017. It is an investigational drug under regulatory review by FDA.
Definition
ChEBI: Vernakalant is an alcohol and a member of phenols.
brand name
Brinavess
Pharmacokinetics
Vernakalant blocks currents in all phases of atrial action potential including atria-specific potassium currents (the ultra-rapid delayed rectifier and the acetylcholine dependent potassium currents) and prolongs the refractory period. It dose-dependently prolongs atrial refractoriness, prolongs AV nodal conduction and refractoriness, and slightly prolongs QRS duration without significantly affecting ventricular refractory period. Vernakalant has a high affinity to ion channels specifically involved in repolarization of atrial tissue and is thought to have a low proarrhythmic potential.
Metabolism
Vernakalant is mainly eliminated by CYP2D6 mediated O-demethylation in CYP2D6 extensive metabolisers. Glucuronidation is the main metabolism pathway in CYP2D6 poor metabolisers.
Properties of Vemakalant
| Boiling point: | 479.0±45.0 °C(Predicted) |
| Density | 1.14±0.1 g/cm3(Predicted) |
| storage temp. | Store at -20°C |
| solubility | DMSO |
| form | Powder |
| pka | 14.79±0.20(Predicted) |
Safety information for Vemakalant
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Vemakalant
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran
You may like
-
![Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%View Details
Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%View Details
61397-56-6 -
 287930-77-2 / 142569-70-8 99%View Details
287930-77-2 / 142569-70-8 99%View Details
287930-77-2 / 142569-70-8 -
![2033-24-1 2,2-Dimethyl-1,3-Dioxane-4,6-Dione [Meldrum Acid] 98%](https://img.chemicalbook.in//Content/image/CP5.jpg) 2033-24-1 2,2-Dimethyl-1,3-Dioxane-4,6-Dione [Meldrum Acid] 98%View Details
2033-24-1 2,2-Dimethyl-1,3-Dioxane-4,6-Dione [Meldrum Acid] 98%View Details
2033-24-1 -
 Ethyl-2-Chloroacetoacetate 609-15-4View Details
Ethyl-2-Chloroacetoacetate 609-15-4View Details
609-15-4 -
 CIS- BROMO BENZOATEView Details
CIS- BROMO BENZOATEView Details
61397-56-6 -
 609-15-4View Details
609-15-4View Details
609-15-4 -
![1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) 1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%View Details
1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%View Details
221615-75-4 -
 27143-07-3View Details
27143-07-3View Details
27143-07-3
