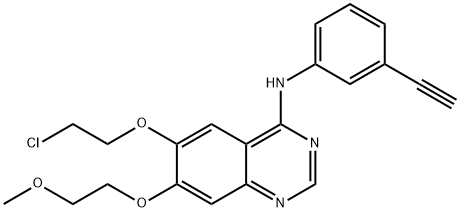
Erlotinib hydrochloride
- CAS NO.:183319-69-9
- Empirical Formula: C22H24ClN3O4
- Molecular Weight: 429.9
- MDL number: MFCD07781272
- EINECS: 620-491-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 21:30:31
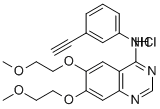
What is Erlotinib hydrochloride ?
Description
Erlotinib, launched as once daily oral treatment for patients with non-small-cell lung cancer (NSCLC), is an inhibitor of the epidermal growth factor receptor (EGFR) tyrosine kinase, and it is the second small-molecule drug to be marketed with this mechanism of action. Both erlotinib and its predecessor, gefitinib, are members of the anilinoquinazoline class of tyrosine kinase inhibitors. They compete with the binding of ATP to the intracellular tyrosine kinase domain of EGFR, thereby inhibiting receptor autophosphorylation and blocking downstream signal transduction.
Chemical properties
Off-White Solid
Originator
Pfizer (US)
The Uses of Erlotinib hydrochloride
Erlotinib Hydrochloride is a HER-1/EGFR tyrosine kinase inhibitor with anti-neoplastic properties, competitive with ATP. Erlotinib hydrochloride (V), a quinazoline derived small molecule inhibitor of epidermal growth factor receptor (EDGFR) tyrosine kinase, was approved in November, 2004, for the treatment of advanced or metastatic non-smallcell lung cancer. It belongs to the same class as gefitinib,another quinazoline approved for treatment of advanced lung cancer, but with improved pharmacokinetic properties. The molecule was originated by Pfizer and development initiated in collaboration with OSI, which assumed full rights to the drug when Pfizer merged with Warner Lambert. Subsequently, Genentech/Roche went into licensing agreement with OSI to develop and market the drug in the US and Worldwide.
The Uses of Erlotinib hydrochloride
Selective epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitor. Antineoplastic
The Uses of Erlotinib hydrochloride
Erlotinib HCl (OSI-744) is an EGFR inhibitor with IC50 of 2 nM, >1000-fold more sensitive for EGFR than human c-Src or v-Abl. Phase 3.
Definition
ChEBI: A quinazoline hydrochloride compound having a (3-ethynylphenyl)amino group at the 4-position and two 2-methoxyethoxy groups at the 6- and 7-positions.
brand name
Tarceva (OSI).
General Description
Erlotinib is available as 25-, 100-, and 150-mg tablets fororal administration and is used after failure of first-linetherapy in metastatic NSCLC and as first-line therapy incombination with gemcitabine in the treatment of metastaticpancreatic cancer, and in treating malignant gliomas.The structural similarity to gefitnib imparts similar pharmacokineticbehavior with bioavailability of 60% and proteinbinding of 93%. The agent is extensively metabolizedprimarily by CYP3A4. Three major metabolic pathwayshave been identified, involving oxidative-O-demethylationof the side chains followed by further oxidation to give thecarboxlic acids, oxidation of the acetylene functionalityto give a carboxylic acid, and aromatic hydroxylation ofthe phenyl ring para to the electron-donating nitrogen. Themetabolites are primarily eliminated in the feces, and theterminal half-life is 36 hours.The major toxicities seenwith the agent are dose-limiting skin rash and diarrhea.Other common adverse effects include shortness of breath,fatigue, and nausea.
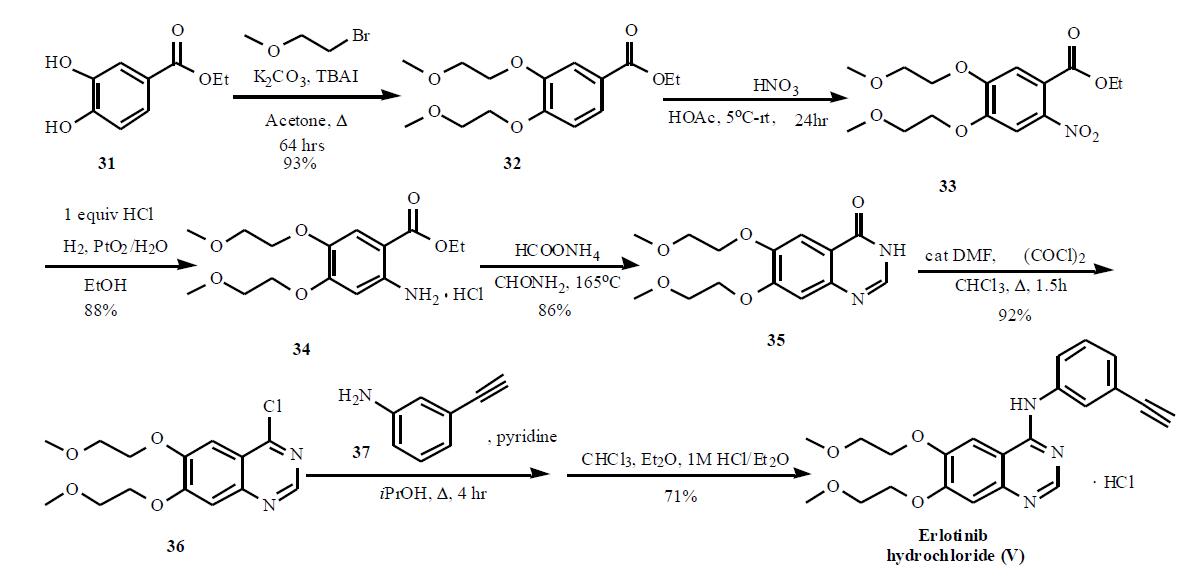
Synthesis
The synthesis of this agent is based on the original patent and is shown in the Scheme. The 3,4-dihydroxy benzoate 31 was reacted with bromoethyl methyl ether in the presence of potassium carbonate and tetrabutyl ammonium iodide to give 32 in 93% yield. Nitration followed by hydrogenation provided 34 in 88% yield, which was then cyclized in formamide with ammonium formate to provide quinazolone 35. Subsequent reaction with oxalyl chloride gave quinazoline chloride 36, which was then reacted with 3-ethynyl aniline (37) in isopropanol in the presence of pyridine to give the desired product erlotinib, which was isolated as the HCl salt (V). An alternate synthesis, that used protected 3-trimethylsilyl ethynyl aniline to couple to the quinazoline chloride 36, has also been published.
in vitro
In vitro, Erlotinib inhibits purified human EGFR tyrosine kinase with an IC50 of 2 nM and blocks EGFR autophosphorylation in cellular assays with an IC50 of 20nM. Treatment of human colon cancer cells with erlotinib was associated with growth inhibition, G1 cell cycle arrest, and apoptosis. Oral administration of erlotinib in athymic mice produced potent antitumor effects with an ED50 of 9.2 mg/kg/day for HN5 head and neck xenografts and 14 mg/ kg/day for A431 epidermoid xenografts.
Storage
Store at -20°C
Production
Erlotinib is prepared by the condensation of 3-ethynylaniline with 4-chloro-6,7-bis(2-methoxyethoxy)quinazoline, which is a key intermediate obtained in five synthetic steps starting from ethyl 3,4- dihydroxybenzoate.
References
1) Moyer et al. (1997), Induction of apoptosis and cell cycle arrest by CP-358,774, an inhibitor of epidermal growth factor tyrosine kinases; Cancer Res., 57 4838 2) Li et al. (2007), Erlotinib effectively inhibits JAK2V617F activity and polycythemia vera cell growth; J. Biol. Chem., 282 3428 3) Wood et al. (2004), A unique structure for epidermal growth factor receptor bound to GW572016 (Lapatinib): relationships among protein conformation, inhibitor off-rate, and receptor activity in tumor cells; Cancer Res., 64 6652 4) Greve et al. (2015), The pan-HDAC inhibitor panobinostat acts as a sensitizer for erlotinib activity in EGFR-mutated and –wildtype non-small cell lung cancer cells; BMC Cancer, 15 947 5) Minquet et al. (2016), Targeted therapies for treatment of non-small cell lung cancer-Recent advances and future perspectives; Int. J. Cancer, 138 2549
Properties of Erlotinib hydrochloride
| Melting point: | 223-225°C |
| storage temp. | Inert atmosphere,Store in freezer, under -20°C |
| solubility | Soluble in DMSO (up to 18 mg/ml with warming). |
| form | Yellow powder. |
| pka | pKa (25°): 5.42 |
| color | White or off-white |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20° for up to 3 months. |
| InChI | InChI=1S/C22H23N3O4.ClH/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22;/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25);1H |
| CAS DataBase Reference | 183319-69-9(CAS DataBase Reference) |
Safety information for Erlotinib hydrochloride
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H413:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. |
Computed Descriptors for Erlotinib hydrochloride
| InChIKey | GTTBEUCJPZQMDZ-UHFFFAOYSA-N |
| SMILES | C12C=C(OCCOC)C(OCCOC)=CC=1N=CN=C2NC1=CC=CC(=C1)C#C.Cl |
Erlotinib hydrochloride manufacturer
ALS India Life Sciences Pvt. Ltd
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 Erlotinib hydrochloride 99%View Details
Erlotinib hydrochloride 99%View Details -
 183319-69-9 Erlotinib hydrochloride 98%View Details
183319-69-9 Erlotinib hydrochloride 98%View Details
183319-69-9 -
 Erlotinib hydrochloride 183319-69-9 99%View Details
Erlotinib hydrochloride 183319-69-9 99%View Details
183319-69-9 -
 Erlotinib Hydrochoride 99%View Details
Erlotinib Hydrochoride 99%View Details -
 ERLOTINIB HCL 183319-69-9 95-99 %View Details
ERLOTINIB HCL 183319-69-9 95-99 %View Details
183319-69-9 -
 Erlotinib HCl (OSI-744) 98% (HPLC) CAS 183319-69-9View Details
Erlotinib HCl (OSI-744) 98% (HPLC) CAS 183319-69-9View Details
183319-69-9 -
 Erlotinib hydrochloride CAS 183319-69-9View Details
Erlotinib hydrochloride CAS 183319-69-9View Details
183319-69-9 -
 N-(3-Ethynylphenyl)-6,7-bis(2-methoxyethoxy)quinazolin-4-amine hydrochloride(Erlotinib HCl) 99.34%View Details
N-(3-Ethynylphenyl)-6,7-bis(2-methoxyethoxy)quinazolin-4-amine hydrochloride(Erlotinib HCl) 99.34%View Details
183319-69-9
