Silica glass
Synonym(s):Silica;Kieselguhr;Monodisperse silicon dioxide;Nanofibrous inorganic powder structure;Non-porous silica
- CAS NO.:60676-86-0
- Empirical Formula: O2Si
- Molecular Weight: 60.0843
- MDL number: MFCD01778719
- EINECS: 262-373-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:22
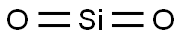
What is Silica glass?
Chemical properties
Made up of spherical submicroscopic particles under 0.1 micron in size.
Chemical properties
Amorphous silica, the noncrystalline form of SiO2, is a transparent to gray, odorless, amorphous powder
Occurrence
This material is known largely as a synthetic material, but there are instances of the material occurring in nature. Vitreous tubes called fulgurites are produced when lightning fuses quartz sand. Large deposits of fulgurite exist in the Libyan desert. Vitreous silica can also be produced by meteor impact. The impact leads to rapid adiabatic heating of the quartz above its melting point. The quartz forms a glass on cooling. Examples of this type of vitreous silica have been found near Canyon Diablo, Arizona, and in meteorite craters in Australia and Arabia.
The Uses of Silica glass
Concrete, grouts, mortars, elastomers, refrac- tory and coating applications.
The Uses of Silica glass
Chemical Applications. Because of its excellent chemical durability, high purity, thermal shock resistance, and usefulness at high temperature, vitreous silica has a wide range of applications in chemical analysis and preparations. Tubing, rods, crucibles, dishes, boats, and other containers and special apparatus are available in both transparent and nontransparent varieties. Because of its inertness, vitreous silica is used as a chromatographic substrate in the form of microparticles, capillary tubing, and open columns for high resolution gas chromatography.
Thermal Applications. The protection of precious-metal thermocouples in high temperature pyrometry is an important application of vitreous silica. Although satin tubing is usually employed, transparent tubes are superior for protecting couples when used in a reducing atmosphere.
Optical Applications. Vitreous silica is ideal for many optical applications because of its excellent uv transmission, resistance to radiation darkening, optical polishing properties, and physical and chemical stability. It is used for prisms, lenses, cells, windows, and other optical components where uv transmission is critical. Cuvettes used in scatter and spectrophotometer cells are manufactured from fused silica and fused quartz because of the transmissive properties and high purity.
Mechanical Applications. The volume of vitreous silica used for fibers is a very small part of the total consumption. However, some interesting and significant applications have been developed in the laboratory, particularly in the area of measurements.
Electronic Applications. In electronic systems, such as radar and computers, signal delay is sometimes necessary. A transducer converts electrical signals to ultrasonic elastic waves, which pass through a connecting medium to another transducer, where the waves are reconverted to electrical signals.
Space and Astronomy. Vitreous silica is used in several space-based applications because of static fatigue (slow crack growth), thermal stability, and radiation resistance. Every U.S. space vehicle having service personnel, including Mercury, Gemini, Apollo, and space shuttle vehicles, has been equipped with windows made of high optical-quality vitreous silica (Corning Code 7940 or 7980) in order to have the clarity needed for visual, photographic, and television-based observations. The space shuttle utilizes triple-layer windows that have outer and central panes of vitreous silica with a tempered aluminosilicate inner pane. The outer pane is thinner for thermal endurance, whereas the two inner panes are thicker to supply strength.
The Uses of Silica glass
Suitable for vacuum deposition.
Production Methods
Modern manufacturing processes of vitreous typically involve the fusion or viscous sintering of silica particles; the particles can be derived from sand crystals or are produced through a chemical process, e.g., flame hydrolysis or sol–gel. In one practice of the flame hydrolysis process, the powder is produced and fused into glass a single step, without the isolation of a porous body. Dopant and additive profiles are concentration are then controlled by the deposition conditions. When a process involving a discrete porous silica body as an intermediate is used, subsequent processing steps can be used to control dopant levels and in particular, the hydroxyl level of the final glass. The choice of fabrication method is often dictated by the end-use specifications. Flame hydrolysis or similar chemical techniques that allow for the production of very high purity glass are the methods of choice for optical applications but may be economically wasteful for less demanding applications.
Translucent Vitreous Silica. Translucent vitreous silica is produced by fusion of high purity quartz sand crystals. Sand is packed around a graphite rod through which a current is passed. The resistance heating produces a plastic mass that can be blown into molds, drawn into tubing, or shaped by rolling or pressing. Separation from the graphite rod is facilitated by gaseous products formed by interfacial reaction. Because the outside is sandy, the product is known as sand-surface ware. A matte finish is obtained by mechanical buffing. A glazed surface is produced by fusing the outside surface with an electric carbon arc or flame.
Transparent Vitreous Silica. Clear, transparent, bubble-free vitreous silica may be obtained by melting natural quartz minerals by flame or plasma vapor deposition (synthetic fused silicas), and by sol–gel processing.
Definition
ChEBI: Silicon dioxide is a silicon oxide made up of linear triatomic molecules in which a silicon atom is covalently bonded to two oxygens.
General Description
Silicon dioxide is one of the important constituents of sedimentary rock bauxite, basalt fibers and ceramic fibers. It is added to cement for improving the hydraulic properties of cement.
Hazard
Questionable carcinogen.
Safety Profile
An inhalation hazard. Questionable carcinogen with experimental tumorigenic data. Poison by intraperitoneal, intravenous, and intratracheal routes. See also other shca entries.
Potential Exposure
Amorphous fumed silica is used as a mineral, natural or synthetic fiber. A potential danger to those involved in the production and handling of fumed silica for paint pigments or catalysts. Diatomaceous earth is used in clarifying liquids, in manufacture of fire brick and heat insulators; used as a filtering agent; as a filler in construction materials; pesticides, paints, and varnishes. A potential danger to those involved in mining of diatomaceous earth or fabrication of products there from.
Purification Methods
Purification of silica for high technology applications uses isopiestic vapour distillation from concentrated volatile acids and is absorbed in high purity water. The impurities remain behind. Preliminary cleaning to remove surface contaminants uses dip etching in HF or a mixture of HCl, H2O2 and deionised water [Phelan & Powell Analyst 109 1299 1984].
Incompatibilities
Silica, amorphous is a noncombustible solid. Generally unreactive chemically. Incompatible with fluorine, oxygen difluoride, chlorine trifluoride. Soluble in molten alkalis and reacts with most metallic oxides at high temperature.
Waste Disposal
Sanitary landfill.
Properties of Silica glass
| Melting point: | 1610 °C(lit.) |
| Boiling point: | 2950°C |
| Density | 2.6 g/mL at 25 °C(lit.) |
| refractive index | n |
| solubility | insoluble in H2O, acid solutions; soluble in HF |
| form | rod (1/8") |
| color | 965 |
| CAS DataBase Reference | 60676-86-0 |
| NIST Chemistry Reference | Silicon dioxide(60676-86-0) |
| EPA Substance Registry System | Silica vitreous (60676-86-0) |
Safety information for Silica glass
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H373:Specific target organ toxicity, repeated exposure |
| Precautionary Statement Codes |
P314:Get medical advice/attention if you feel unwell. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Silica glass
Silica glass manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 Silicon dioxide,acid purified, sand CAS 60676-86-0View Details
Silicon dioxide,acid purified, sand CAS 60676-86-0View Details
60676-86-0 -
 Silicon dioxide, -325 mesh CASView Details
Silicon dioxide, -325 mesh CASView Details -
 Quartz single crystal substrate, <0001> CAS 60676-86-0View Details
Quartz single crystal substrate, <0001> CAS 60676-86-0View Details
60676-86-0 -
 Silicon dioxide, ≥99.99% CASView Details
Silicon dioxide, ≥99.99% CASView Details -
 Silicon dioxide CAS 60676-86-0View Details
Silicon dioxide CAS 60676-86-0View Details
60676-86-0 -
 BRAND® filter funnel support with base plate CASView Details
BRAND® filter funnel support with base plate CASView Details -
 BRAND® filter funnel support with base plate CASView Details
BRAND® filter funnel support with base plate CASView Details -
 Celite 545View Details
Celite 545View Details
60676-86-0
