Fumed Silica
Synonym(s):Silica;Silicon dioxide;Silica gel;Quartz;Sand
- CAS NO.:112945-52-5
- Empirical Formula: O2Si
- Molecular Weight: 60.08
- MDL number: MFCD01778716
- EINECS: 231-545-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
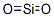
What is Fumed Silica?
Chemical properties
Amorphous silica, the noncrystalline form of SiO2, is a transparent to gray, odorless, amorphous powder
Chemical properties
Colloidal silicon dioxide is a submicroscopic fumed silica with a particle size of about 15 nm. It is a light, loose, bluish-white-colored, odorless, tasteless, amorphous powder.
The Uses of Fumed Silica
Synthetic Amorphous Silica has interesting thickening and thixotropic properties, and an enormous external surface area. It is produced by a vapor phase hydrolysis process using chlorosilanes or substituted silanes such as, silicon tetrachloride in a flame of hydrogen and oxygen. This material is formed and collected in a dry state. This product contains no detectable crystalline silica.
What are the applications of Application
Silica, fumed is A chemical used in the synthesis of silicalite and in the catalytic growth of oxide and nitride nanowires.2
Production Methods
Colloidal silicon dioxide is prepared by the flame hydrolysis of chlorosilanes, such as silicon tetrachloride, at 18008℃ using a hydrogen–oxygen flame. Rapid cooling from the molten state during manufacture causes the product to remain amorphous.
Definition
ChEBI: Silicon dioxide is a silicon oxide made up of linear triatomic molecules in which a silicon atom is covalently bonded to two oxygens.
General Description
Fumed silica may be synthesized by high temperature hydrolysis of SiCl4 in O2(N2)/H2 flame. It is amorphous in nature and possesses very high specific area. The micro droplets of amorphous silica fuse into a branch and form a chain like agglomerate.
Pharmaceutical Applications
Colloidal silicon dioxide is widely used in pharmaceuticals,
cosmetics, and food products. Its small particle size
and large specific surface area give it desirable flow characteristics
that are exploited to improve the flow properties of dry powders
in a number of processes such as tableting and capsule filling.
Colloidal silicon dioxide is also used to stabilize emulsions and
as a thixotropic thickening and suspending agent in gels and
semisolid preparations. With other ingredients of similar refractive
index, transparent gels may be formed. The degree of viscosity
increase depends on the polarity of the liquid (polar liquids
generally require a greater concentration of colloidal silicon dioxide
than nonpolar liquids). Viscosity is largely independent of
temperature. However, changes to the pH of a system may affect
the viscosity1.
In aerosols, other than those for inhalation, colloidal silicon
dioxide is used to promote particulate suspension, eliminate hard
settling, and minimize the clogging of spray nozzles. Colloidal
silicon dioxide is also used as a tablet disintegrant and as an
adsorbent dispersing agent for liquids in powders. Colloidal
silicon dioxide is frequently added to suppository formulations
containing lipophilic excipients to increase viscosity, prevent
sedimentation during molding, and decrease the release rate.
Colloidal silicon dioxide is also used as an adsorbent during the
preparation of wax microspheres; as a thickening agent for
topical preparations; and has been used to aid the freeze-drying
of nanocapsules and nanosphere suspensions.
Safety Profile
Poison by intraperitoneal, intravenous, and intratracheal routes. Moderately toxic by ingestion. An inhalation hazard. Much less toxic than crystalhe forms. Questionable carcinogen with experimental carcinogenic data. Mutation data reported. Does not cause silicosis. See also other silica entries
Safety
Colloidal silicon dioxide is widely used in oral and topical
pharmaceutical products and is generally regarded as an essentially
nontoxic and nonirritant excipient. However, intraperitoneal and
subcutaneous injection may produce local tissue reactions and/or
granulomas. Colloidal silicon dioxide should therefore not be
administered parenterally.
LD50 (rat, IV): 0.015 g/kg
LD50 (rat, oral): 3.16 g/kg
Potential Exposure
Amorphous fumed silica is used as a mineral, natural or synthetic fiber. A potential danger to those involved in the production and handling of fumed silica for paint pigments or catalysts. Diatomaceous earth is used in clarifying liquids, in manufacture of fire brick and heat insulators; used as a filtering agent; as a filler in construction materials; pesticides, paints, and varnishes. A potential danger to those involved in mining of diatomaceous earth or fabrication of products there from.
storage
Colloidal silicon dioxide is hygroscopic but adsorbs large quantities of water without liquefying. When used in aqueous systems at a pH 0–7.5, colloidal silicon dioxide is effective in increasing the viscosity of a system. However, at a pH greater than 7.5 the viscosityincreasing properties of colloidal silicon dioxide are reduced; and at a pH greater than 10.7 this ability is lost entirely since the silicon dioxide dissolves to form silicates. Colloidal silicon dioxide powder should be stored in a well-closed container.
Purification Methods
Purification of silica for high technology applications uses isopiestic vapour distillation from concentrated volatile acids and is absorbed in high purity water. The impurities remain behind. Preliminary cleaning to remove surface contaminants uses dip etching in HF or a mixture of HCl, H2O2 and deionised water [Phelan & Powell Analyst 109 1299 1984].
Incompatibilities
Silica, amorphous is a noncombustible solid. Generally unreactive chemically. Incompatible with fluorine, oxygen difluoride, chlorine trifluoride. Soluble in molten alkalis and reacts with most metallic oxides at high temperature.
Incompatibilities
Incompatible with diethylstilbestrol preparations.
Waste Disposal
Sanitary landfill.
Regulatory Status
GRAS listed. Included in the FDA Inactive Ingredients Database (oral capsules, suspensions, and tablets; transdermal, rectal, and vaginal preparations). Also approved by the FDA as a food additive and for food contact. Included in nonparenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Nonmedicinal Ingredients.
Properties of Fumed Silica
| Melting point: | >1600°C |
| Density | 2.3 lb/cu.ft at 25 °C (bulk density)(lit.) |
| refractive index | n |
| solubility | Practically insoluble in organic solvents, water, and
acids, except hydrofluoric acid; soluble in hot solutions of alkali
hydroxide. Forms a colloidal dispersion with water. For Aerosil,
solubility in water is 150 mg/L at 258℃ (pH 7). |
| form | powder |
| Specific Gravity | 2.2 |
| Hydrolytic Sensitivity | 5: forms reversible hydrate |
| CAS DataBase Reference | 112945-52-5(CAS DataBase Reference) |
| EPA Substance Registry System | Silica, amorphous, fumed, cryst.-free (112945-52-5) |
Safety information for Fumed Silica
Computed Descriptors for Fumed Silica
Fumed Silica manufacturer
Bazayan & Co.
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran








You may like
-
 10279-57-9 / 12508-66-6 98%View Details
10279-57-9 / 12508-66-6 98%View Details
10279-57-9 / 12508-66-6 -
 Silica, 99.8% CAS 112945-52-5View Details
Silica, 99.8% CAS 112945-52-5View Details
112945-52-5 -
 Silica Gel Fumed 200 (Hydrophilic) CAS 112945-52-5View Details
Silica Gel Fumed 200 (Hydrophilic) CAS 112945-52-5View Details
112945-52-5 -
 Silica, fumed, nanopowder CAS 112945-52-5View Details
Silica, fumed, nanopowder CAS 112945-52-5View Details
112945-52-5 -
 Silica fumed, fine powder CAS 112945-52-5View Details
Silica fumed, fine powder CAS 112945-52-5View Details
112945-52-5 -
 Cabosil CASView Details
Cabosil CASView Details -
 Silica, fumed CAS 112945-52-5View Details
Silica, fumed CAS 112945-52-5View Details
112945-52-5 -
 Silica CAS 112945-52-5View Details
Silica CAS 112945-52-5View Details
112945-52-5
