Safinamide
- CAS NO.:133865-89-1
- Empirical Formula: C17H19FN2O2
- Molecular Weight: 302.34
- MDL number: MFCD00897036
- EINECS: 603-772-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-08-06 15:14:14
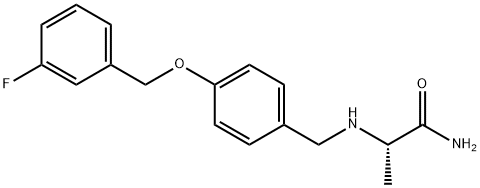
What is Safinamide?
Absorption
Rapid with peak plasma concentrations ranging from 2 to 4 h, total bioavailability is 95%. Food prolonged the rate and did not affect the extent of absorption of safinamide.
Toxicity
uncontrolled involuntary movement, falls, nausea, and trouble sleeping or falling asleep (insomnia) Patients who have an overdose may experience hypertension (high blood pressure), orthostatic hypotension, hallucinations, psychomotor agitation, nausea, vomiting, and dyskinesia.
Description
Safinamide (133865-89-1) is a selective and reversible inhibitor of monoamine oxidase B (MAO-B, IC50=98 nM with greater than 100-fold selectivity over MAO-A.1?Displays anticonvulsant activity2?and protects against kainate-induced seizures and hippocampal neurodegeneration in rat models3. Reduces overactive glutamatergic signaling via use-dependent sodium channel blockade.4?A novel therapeutic for Parkinson’s disease with multiple modes of action.5?Effective as an add-on to dopamine agonist therapy in early Parkinson’s.6
History
Safinamide is an α-aminoamide derivative with a multimodal mechanism of action involving both dopaminergic and non-dopaminergic properties. It increases levels of available dopamine through dopamine reuptake inhibition andMAO-B inhibition. It is a potent and reversible inhibitor of MAO-B, with significantly greater selectivity for MAO-B over MAO-A than selegiline and rasagiline. However, it also has an important and novel mode of action that involves blockade of Na+ channels andmodulation of Ca2+ channels that inhibits glutamate release and therefore may provide a level of cognitive improvement and neuroprotection. Homeostatic interactions between dopamine and glutamate are central to the normal physiology of the basal ganglia. In PD, this relationship is altered, resulting in upregulation of cortico-striatal glutamatergic function in l-dopa-induced dyskinesia. Any drug that can counteract such unbalance in glutamate function is potentially useful in controlling dyskinesias.
Safinamide was originated by Farmitalia Carlo Erba, a company that was later purchased by Pharmacia. Newron Pharmaceuticals was established as a spin-off of Pharmacia in 1999 and acquired safinamide rights and intellectual property from Pharmacia Corporation. Newron originally granted Serono exclusive worldwide rights to develop, manufacture, and commercialize safinamide in 2006. However, in October 2011, Merck Serono agreed to return full global rights for safinamide to Newron. Newron then finalized a strategic collaboration and license agreement with Zambon for the worldwide development and commercialization of safinamide.
Indications
Safinamide is indicated as an add-on treatment to levodopa with or without other medicines for Parkinson’s disease
Background
Safinamide is for the treatment of parkinson's disease. It was approved in Europe in February 2015, and in the United States on March 21, 2017.
Indications
In 2003 positive preliminary results from a phase II trial of safinamide in epilepsy were announced. This open-labeled study was initiated to assess tolerability and drug–drug interaction (DDI) of safinamide in 48 patients with uncontrolled seizures that had already been treated with up to three other antiepileptic drugs. Starting with an initial oral dose of 50mg d−1, safinamide was increased every two weeks up to 300 mg d−1 or to the maximum tolerated dose. An interim analysis of the first 29 patients who completed the study showed excellent tolerability. In this group, no DDI was noted at any of the tested doses in that safinamide did not alter the kinetics of the other antiepileptic drugs. In this interim analysis safinamide was shown to be well tolerated in patients with medically intractable seizures. No serious AEs occurred in the study. Even though the study was not designed and powered to provide proof of efficacy, the sponsor reported that preliminary data showed a significant reduction in median seizure frequency from 50mg progressing up to the highest dose.
In 2005 safinamide was administered to a total of 10 patients with RLS that were enrolled into a single-center, phase II open-labeled pilot study. Each patient was administered safinamide (100 mg d−1) at bedtime for 2 weeks. A significant improvement in all efficacy parameters studied was observed when patients received safinamide. RLS is a neurological disorder characterized by jerky movements of the lower extremities that appear mostly in the evening and during sleep. As reported in a press release, safinamide was also found to be well tolerated and did not exhibit any clinically relevant side effects, but no study results have been published.
Definition
ChEBI: Safinamide is an amino acid amide.
Mechanism of action
Safinamide may exert its in vivo effects through different mechanisms of action. It did not display activity against >80 different types of dopamine, glutamate, adenosine, serotonin, muscarinic, nicotinic, and GABA receptors. Conversely, potent modulation of DA metabolism, blockade of Na+/Ca2+ channels, and inhibition of glutamate release have been demonstrated. It has pointed out that the electrophysiological and neurochemical effects of safinamide are apparent at effective anticonvulsant concentrations. For example, pharmacokinetics (PK) showed that brain levels reach roughly 40 μM, at 30 and 60min after an oral dose of 10 mg kg?1 safinamide in rats. These points in time correspond to the peak anticonvulsant effect observed in the MES epilepsy model. This micromolar concentration approximates the concentration of safinamide that gives effective inhibition of excitatory amino acid release and reduction of sustained repetitive firing (SRF). Importantly, safinamide partitions itself well into the brain, where drug levels are approximately 10-fold higher than in plasma.
Clinical Use
Highly selective and reversible MAO-B inhibitor:
Treatment of Parkinson’s disease
Drug interactions
Potentially hazardous interactions with other drugs
Analgesics: avoid with pethidine.
Antibacterials: possible enhanced hypotensive effect
with linezolid and tedizolid.
Antidepressants: increased risk of hypertension
and CNS excitation with SSRIs and tricyclics -
adjust SSRI or tricyclic doses, avoid or adjust dose
of fluoxetine and fluvoxamine; possible enhanced
hypotensive effect with MAOIs and moclobemide -
avoid.
Sympathomimetics: use with caution.
Metabolism
The principal step is mediated by amidases which have not been identified, and produces safinamide acid. It is also metabolized to O-debenzylated safinamide and N-delkylated amine. The N-dealkylated amine is then oxidized to a carboxylic acid and finally glucuronidated. Dealkylation reactions are mediated by cytochrome P450s (CYPs), especially CYP3A4. Safinamide acid binds to organic anion transporter 3 (OAT3), but no clinical relevance of this interaction has been determined. Safinamide also binds to ABCG2 transiently. No other transporter affinities have been found in preliminary studies.
Metabolism
There are three routes of hepatic metabolism. The main
route involves hydrolytic oxidation of the amide moiety
leading to the main metabolite safinamide acid (NW-
1153). Another pathway involves oxidative cleavage of
the ether bond forming 'O-debenzylated safinamide'
(NW-1199). Finally the 'N-dealkylated acid' (NW-1689)
is formed by oxidative cleavage of the amine bond of
either safinamide (minor) or the primary safinamide acid
metabolite (NW-1153) (major). The 'N-dealkylated acid'
(NW-1689) undergoes conjugation with glucuronic acid
yielding its acyl glucuronide. None of these metabolites
are pharmacologically active.
In humans, safinamide is almost exclusively eliminated via
metabolism of which 76% is renal and 1.5% via the faeces.
References
1) Strolin Benedetti?et al.?(1994),?The anticonvulsant FCE 26743 is a selective and short-acting MAO-B inhibitor devoid of inducing properties towards cytochrome P450-dependent testosterone hydroxylation in mice and rats;?J. Pharm. Pharmacol.,?46?814 2) Fariello?et al.?(1998),?Preclinical evaluation of PNU-151774E as a novel anticonvulsant; J. Pharmacol. Exp. Ther.?285?397 3) Maj?et al.?(1998),?PNU-151774E protects against kainate-induced status epilepticus and hippocampal lesions in the rat; Eur, J. Pharmacol.,?59?27 4) Gardoni?et al.?(2018),?Safinamide Modulates Striatal glutamatergic Signaling in a Rat Model of Levodopa-Induced Dyskinesia; J. Pharmacol. Exp. Ther., 367?442 5) Caccia?et al.?(2006),?Safinamide: from molecular targets to a new anti-Parkinson drug; Neurology,?67(7 Suppl. 2)?S18 6) Schapira?et al.?(2013),?Long-term efficacy and safety of safinamide as add-on therapy in early Parkinson’s disease; Eur. J. Neurol.,?20?271
Properties of Safinamide
| Melting point: | 208-212° |
| Boiling point: | 476.7±40.0 °C(Predicted) |
| Density | 1.189±0.06 g/cm3(Predicted) |
| storage temp. | under inert gas (nitrogen or Argon) at 2–8 °C |
| solubility | Soluble in DMSO (up to 50 mg/ml). |
| form | solid |
| pka | 16.03±0.50(Predicted) |
| color | White |
| Merck | 14,8315 |
| Stability: | Stable for 2 years from date of purchase as supplied. Solutions in DMSO may be stored at -20° for up to 3 months. |
Safety information for Safinamide
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Safinamide
Safinamide manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

You may like
-
 Safinamide 99%View Details
Safinamide 99%View Details -
 133865-89-1 98%View Details
133865-89-1 98%View Details
133865-89-1 -
 133865-89-1 Safinamide 98%View Details
133865-89-1 Safinamide 98%View Details
133865-89-1 -
 Safinamide CAS 133865-89-1View Details
Safinamide CAS 133865-89-1View Details
133865-89-1 -
 SafinamideView Details
SafinamideView Details
133865-89-1 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
