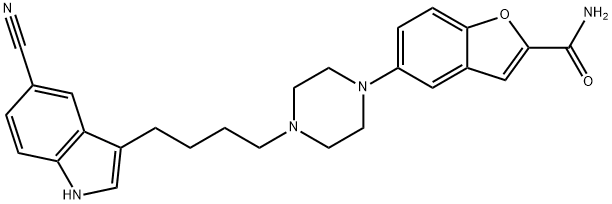
VILAZODONE
- CAS NO.:163521-12-8
- Empirical Formula: C26H27N5O2
- Molecular Weight: 441.52
- MDL number: MFCD09838919
- EINECS: 1308068-626-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-16 16:15:04
What is VILAZODONE?
Absorption
Vilazodone's bioavailability is 72% when taken with food.
Toxicity
There is a lack of clinical studies of vilazodone in pregnancy. Animal studies have shown the effects on offspring to be reduced fetal weight, increased mortality, delayed maturation, and decreased fertility in adulthood at doses well above the maximum recommended human dose. Clinical cases of fetal and neonatal exposure to SSRIs and SNRIs have lead to a number of complications including respiratory distress, seizures, and temperature instability. It is not know whether vilazodone is excreted in the breast milk of nursing mothers but animal studies show this is the case for rats. The risk and benefit of breast feeding while taking vilazodone for mother and child must be considered before a decision is made. Safety and effectiveness in pediatric patients has not been established in clinical trials though antidepressants are associated with an increased risk of suicidal thought and behaviour in patients under 24 years. Clinical studies in geriatric patients showed to significant difference in response to vilazodone compared to younger patients. Geriatric patients should be started at a lower dose and titrated to an effective dose as they are more likely to have other comorbidities. Dosage adjustments are not necessary for patients of different genders or with reduced hepatic and renal function.
Description
In January 2011, the U.S. FDA approved vilazodone for the treatment of major depressive disorder (MDD). Vilazodone is a novel antidepressant agent that combines potent serotonin reuptake inhibition (IC50=0.2 nM) with high affinity for 5-HT1A receptors (IC50=0.5 nM)and partial 5-HT1A receptor agonist functional activity. Vilazodone has good selectivity over other monoamine receptors and is efficacious in preclinical models of depression in rats and mice. Vilazodone is an indolylbutylpiperazine derivative that has been prepared by coupling of an indolylbutyl chloride or tosylate with 5-(piperazin-1-yl)benzofuran-2- carboxamide or the corresponding ester. The cyanoindole portion of vilazodone is important for conferring high affinity for both the serotonin transporter and the 5-HT1A receptor. Para-substitution on the phenyl group attached to the piperidine moiety reduces affinity for dopamine receptors, while the carboxamide group provides improved pharmacokinetic properties.
Chemical properties
Light Yellow Solid
Originator
Merck KGaA (Germany)
The Uses of VILAZODONE
Labelled analogue of Vilazodone
The Uses of VILAZODONE
It is a combined serotonin specific reuptake inhibitor (SSRI) and 5-HT1A receptor partial agonist currently under clinical evaluation for the treatment of major depression.
The Uses of VILAZODONE
An inhibitor of ST and activator of SR-1A
What are the applications of Application
Vilazodone is Vilazodone is an inhibitor of ST and activator of SR-1A
Indications
Vilazodone is approved for treatment of major depressive disorder.
Background
Vilazodone is a novel compound with combined high affinity and selectivity for the 5-hydroxytryptamine (5-HT) transporter and 5-HT(1A) receptors. Vilazodone may also be associated with less sexual dysfunction and weight gain. Vilazodone was given FDA approval on January 21, 2011.
Definition
ChEBI: A 1-benzofuran that is 5-(piperazin-1-yl}-1-benzofuran-2-carboxamide having a (5-cyanoindol-3-yl)butyl group attached at position N-4 on the piperazine ring. Used for the treatment of major depressive disorder.
brand name
Viibryd
Pharmacokinetics
Vilazodone increases serotonin levels in the brain by inhibiting the reuptake of serotonin while acting as a partial agonist on serotonin-1A receptors. Due to this activity vilazodone has sometimes been referred to as a selective partial agonist and reuptake inhibitor (SPARI).
Metabolism
Vilazodone is mainly metabolized by cytochrome P450(CYP)3A4 and also to a minor extent by CYP2C19 and CYP 2D6. Although the metabolic pathway for vilazodone has not been fully studied, a proposed mechanism for metabolism in rats was published in 2017.
Properties of VILAZODONE
| Melting point: | 203-205°C |
| Boiling point: | 745.1±60.0 °C(Predicted) |
| Density | 1.34±0.1 g/cm3(Predicted) |
| storage temp. | Keep in dark place,Sealed in dry,Store in freezer, under -20°C |
| solubility | DMSO (Slightly)), Methanol (Slightly) |
| form | Solid |
| pka | 15.98±0.30(Predicted) |
| color | Pale Yellow to Beige |
| Stability: | Very Hygroscopic |
| CAS DataBase Reference | 163521-12-8 |
Safety information for VILAZODONE
Computed Descriptors for VILAZODONE
VILAZODONE manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 163521-12-8 98%View Details
163521-12-8 98%View Details
163521-12-8 -
 Vilazodone 163521-12-8 98%View Details
Vilazodone 163521-12-8 98%View Details
163521-12-8 -
 163521-12-8 Vilazodone 98%View Details
163521-12-8 Vilazodone 98%View Details
163521-12-8 -
 Vilazodone 98%View Details
Vilazodone 98%View Details
163521-12-8 -
 Vilazodone 163521-12-8 99%View Details
Vilazodone 163521-12-8 99%View Details
163521-12-8 -
 163521-12-8 VILAZODONE HCl 95-99 %View Details
163521-12-8 VILAZODONE HCl 95-99 %View Details
163521-12-8 -
 Vilazodone 95% CAS 163521-12-8View Details
Vilazodone 95% CAS 163521-12-8View Details
163521-12-8 -
 Vilazodone Powder APIView Details
Vilazodone Powder APIView Details
163521-12-8
