Rasagiline
- CAS NO.:136236-51-6
- Empirical Formula: C12H13N
- Molecular Weight: 171.24
- MDL number: MFCD00866571
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
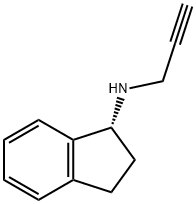
What is Rasagiline?
Absorption
Rasagiline is rapidly absorbed following oral administration. The absolute bioavailability of rasagiline is about 36%.
Toxicity
Signs and symptoms of overdosage may include, alone or in combination, any of the following: drowsiness, dizziness, faintness, irritability, hyperactivity, agitation, severe headache, hallucinations, trismus, opisthotonos, convulsions, and coma; rapid and irregular pulse, hypertension, hypotension and vascular collapse; precordial pain, respiratory depression and failure, hyperpyrexia, diaphoresis, and cool, clammy skin.
Description
Rasagiline is a second-generation, irreversible monoamine oxidase type B (MAO-B) inhibitor that has been launched for the treatment of Parkinson’s disease (PD). Unlike its predecessor selegiline, it is not metabolized to amphetamine derivatives and is, therefore, devoid of the sympathomimetic activity responsible for adverse side effects. Rasagiline is, however, similar to selegiline in the retention of the propargylamine moiety; this essential pharmacophore binds covalently to selectively form an irreversible bond with the flavin adenine dinucleotide portion of the MAO-B enzyme. As an adjunct therapy, rasagiline treats the fluctuations in motor symptoms. The R-enantiomer exhibits 4-times the potency of the S-enantiomer, so the synthetic method begins with the optical resolution of racemic N-benzyl-1-aminoindan using (R,R)-tartaric acid as the resolving agent. Once isolated, the enantiomerically-enriched salt is submitted to hydrogenolysis to afford 1(R)- aminoindane that is subsequently propargylated to provide rasagiline. It is formulated as its mesylate salt, and the recommended dosage of rasagiline is 1 mg/day, with or without levodopa. Entacapone, a catecholamine- O-methyltransferase inhibitor known as an effective add-on therapy for motor fluctuations, was used as a comparator. Rasagiline reduced the time spent in the “off” state while increasing the “on” time.
Originator
Teva/Eisai/Lundbeck (Israel)
Characteristics
Rasagiline, [N-propargyl-1R(+)aminoindan] is a unique, selective, and potent secondgeneration mitochondrial monoamine oxidase B inhibitor with distinctive neuroprotective as well as therapeutic properties for the treatment of PD.
The Uses of Rasagiline
5HT4 receptor agonist, peristaltic stimulant. Rasagiline, is a selective and irreversible propargylamine inhibitor of monoamine oxidase which has been used to increase the availability of dopamine at striatal receptors as a method to treat Parkinson’s disease.
Indications
For the treatment of the signs and symptoms of idiopathic Parkinsons disease as initial monotherapy and as adjunct therapy to levodopa.
Background
Rasagiline is an irreversible inhibitor of monoamine oxidase and is used as a monotherapy in early Parkinson's disease or as an adjunct therapy in more advanced cases.
What are the applications of Application
Rasagiline is an irreversible inhibitor of monoamine oxidase
Definition
ChEBI: An indane that consists of 1-aminoindane bearing an N-propargyl substituent. A selective, irreversible monoamine oxidase-B inhibitor.
Indications
Rasagiline has a role in the treatment of PD by virtue of its proven ability to reduce the signs of PD in both the “on” and “off” states, and to improve global function. It appears to be of value in early stages of PD as well as after the appearance of clinical fluctuations in response to LD. Rasagiline also has a promising but not fully explored potential to halt or slow down the progression of PD, as well as other clinical conditions. The accumulating evidence of rasagiline’s neuroprotective effects in animal and cellular models is both intriguing and exciting. Further careful scientific basic and clinical studies and clinical experience are needed to establish the full therapeutic benefits of rasagiline for the treatment of PD.
What are the applications of Application
Rasagiline is used alone or with other medications (such as levodopa/carbidopa) to treat symptoms of Parkinson's disease. It can help improve symptoms such as shakiness, stiffness, and difficulty moving. It can also help reduce the amount of "off" time (periods of slow movement or stiffness). Rasagiline belongs to a class of drugs known as MAO inhibitors. It works by increasing the levels of certain natural substances in the brain (such as dopamine, norepinephrine, serotonin). Parkinson's disease is thought to be caused by too little dopamine in the brain.
brand name
Azilect (Teva).
Mechanism of action
Rasagiline has many neuroprotective properties in animal and cell culture models of PD as well as a variety of other conditions that are too numerous to delineate completely, so only some of these studies are mentioned here.
Some studies suggest that the neuroprotective effects of rasagiline are potentially separate and unrelated to its therapeutic effects.For example, the S-enantiomer of rasagiline lacks the MAOB inhibitory and the antidepressant effects of rasagiline but shares with rasagiline its neuroprotective effects and its cholinesterase inhibitory effects.This suggests that the neuroprotective effects of rasagiline are independent of its antidepressant and MAOB inhibitory effects. Rasagiline protects dopaminergic SHSY5Y cells in culture by inhibiting mitochondrial permability transition, an apoptosis inducing effect, of an endogenous neurotoxin (N-methyl(R) salsolinol).In the same cell model, rasagiline also enhances the expression of the Bcl-2 gene family which have antiapoptotic effects.Finally, rasagiline prevents the apoptosis and nuclear accumulation of glyceraldehyde-3-phosphate dehydrogenase induced by N-methyl (R) salsonilol in human dopaminergic SH-SY5Y cell cultures.Since the SH-SY5Y cell system does not contain MAO, these protective effects must have other mechanisms. Rasagiline also is protective in vivo against MPTP (mice) and 6OHDA (rats) (see Reference 100). In primate models of PD, rasagiline led to a return toward normal of cognitive and motor function, even in the absence of LD.
In neuronal cell models (SH-SY5Y neuroblastoma cells and neuronally differentiated PC12 cells), rasagiline increases SOD activity and Bcl-2 expression, and it inhibits activation of capsase 3, prevents DNA laddering, inhibits toxin induced fall in mitochondrial membrane potential, prevents toxin induced apoptosis, and protects against cell death due to ischemia and glucose deprivation.Rasagiline also has protective effects against stroke occurrence and promotes survival in spontaneously hypertensive rats.
Pharmacokinetics
Rasagiline is a propargylamine and an irreversible inhibitor of monoamine oxidase (MAO). MAO, a flavin-containing enzyme, regulates the metabolic degradation of catecholamines and serotonin in the CNS and peripheral tissues. It is classified into two major molecular species, A and B, and is localized in mitochondrial membranes throughout the body in nerve terminals, brain, liver and intestinal mucosa. MAO-A is found predominantly in the GI tract and liver, and regulates the metabolic degradation of circulating catecholamines and dietary amines. MAO-B is the major form in the human brain and is responsible for the regulation of the metabolic degradation of dopamine and phenylethylamine. In ex vivo animal studies in brain, liver and intestinal tissues rasagiline was shown to be a potent,selective, and irreversible monoamine oxidase type B (MAO-B) inhibitor. At the recommended therapeutic doses, Rasagiline was also shown to be a potent and irreversible inhibitor of MAO-B in platelets. The selectivity of rasagiline for inhibiting only MAO-B (and not MAO-A) in humans and the sensitivity to tyramine during rasagiline treatment at any dose has not been sufficiently characterized to avoid restriction of dietary tyramine and amines contained in medications.
Pharmacokinetics
Rasagiline, or TV3326, or [N-propargyl-1R(+)ami-noindan], is a selective and highly potent second-generation mitochondrial monoamine oxidase B inhibitor.As opposed to selegiline, rasagiline has quite a different metabolite profile. Selegiline’s major metabolites are amphetamine and methamphetamine, while rasagiline’s primary metabolite is aminoindan. While amphetamine and methamphetamine are potently addictive substances, they may promote alertness. Aminoindan has beneficial effects of its own and has no known adverse side effects.In man, 1-mg daily dosages of rasagiline inhibit platelet MAO-B nearly completely.Rasagiline has both therapeutic and protective properties.
Clinical Use
Rasagiline [R(+)-N-propargyl-1-aminoindan] mesylate (Azilect®) was approved by the FDAin May of 2006 as monotherapy in early disease and as an adjunct to levodopa in more advanced disease. The recommended doses are 1 mg once a day in early disease and an initial dose of 0.5 mg once a day in advanced disease that can be increased to 1 mg once a day if needed. It produces selective irreversible MAO-B inhibition. Platelet MAO-B inhibition is dose-dependent; one hour after ingestion, platelet MAOB inhibition is 35% with 1 mg rasagiline and 99% with 10 mg rasagiline. By day 6, rasagiline 2 mg/day inhibits over 99% of platelet MAO-B. After discontinuing rasagiline, it takes approximately two weeks for MAO-B activity to return to baseline values. The area under the curve (AUC) and maximum concentration (Cmax) increase linearly with rasagiline dosage. The plasma half-lives of rasagiline and its active metabolite 1(R)-aminoindan are 3.5 hours and 11 hours, respectively. As rasagiline irreversibly inhibits MAO-B, the serum (pharmacokinetic) half-life does not correlate with its functional (pharmacodynamic) half-life.
Rasagiline up to 20 mg/day was well tolerated in healthy male volunteers. Dry mouth, headache, nausea, thirst, and abdominal discomfort were the most common adverse effects but tended to be mild. There were no significant effects on vital signs, lab values, physical exam, or EKG.
Side Effects
Common side effects of Rasagiline may occur as dizziness, drowsiness, joint pain, heartburn, nausea, dry mouth, weight loss or stomach/abdominal pain. Severe fainting, loss of balance, mental/mood changes (e.g. confusion, depression, hallucinations), muscle stiffness/twitching/uncontrollable movements, swelling of ankles/legs, easy bleeding/bruising, abnormally strong impulses (e.g. increased gambling, increased sexual urges) may occur. It may increase serotonin and rarely causes a very serious condition called serotonin syndrome/toxicity. Very rarely an allergic reaction may occur.
Drug interactions
Potentially hazardous interactions with other drugs
Analgesics: avoid with dextromethorphan; avoid
with pethidine (risk of serious adverse reactions) -
allow at least 14 days before starting pethidine.
Antidepressants: avoid with other MAOIs (can
lead to hypertensive crisis) - allow at least 14 days
before starting a MAOI; avoid with fluoxetine
and fluvoxamine; allow 5 weeks between stopping
fluoxetine and starting rasagiline; allow 14 days
between stopping rasagiline and starting fluoxetine
or fluvoxamine; increased CNS toxicity with SSRIs,
tricyclics and vortioxetine.
Sympathomimetics: concomitant use is not
recommended.
Metabolism
Rasagiline undergoes almost complete biotransformation in the liver prior to excretion. In vitro experiments indicate that both routes of rasagiline metabolism are dependent on the cytochrome P450 (CYP) system, with CYP 1A2 being the major isoenzyme involved in rasagiline metabolism.
Metabolism
Rasagiline is extensively metabolised in the liver by
N-dealkylation and hydroxylation, via the cytochrome
P450 isoenzyme CYP1A2, and conjugation.
1-Aminoindan is a major metabolite and is stated to be
active although it is not a monoamine oxidase B inhibitor.
The metabolites are excreted mainly in the urine and
partly in the faeces.
Properties of Rasagiline
| Melting point: | 148 °C |
| Boiling point: | 305.5±30.0 °C(Predicted) |
| Density | 1.05±0.1 g/cm3(Predicted) |
| storage temp. | under inert gas (nitrogen or Argon) at 2–8 °C |
| solubility | Dichloromethane |
| form | Solid |
| pka | 6.95±0.20(Predicted) |
| color | Off-White to Light Beige to Orange Sticky to |
| Stability: | Light Sensitive |
| InChI | InChI=1S/C12H13N/c1-2-9-13-12-8-7-10-5-3-4-6-11(10)12/h1,3-6,12-13H,7-9H2/t12-/m1/s1 |
Safety information for Rasagiline
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Rasagiline
| InChIKey | RUOKEQAAGRXIBM-GFCCVEGCSA-N |
| SMILES | [C@H]1(NCC#C)C2=C(C=CC=C2)CC1 |
Rasagiline manufacturer
Dayaram Pharma Chem
BDR Pharmaceuticals International Pvt Ltd
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

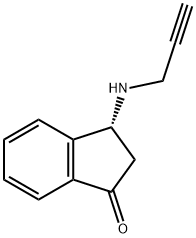

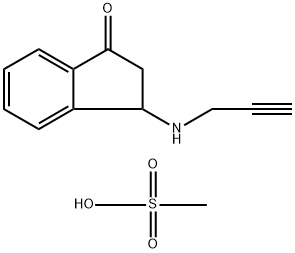
You may like
-
 136236-51-6 Rasagiline 98%View Details
136236-51-6 Rasagiline 98%View Details
136236-51-6 -
 136236-51-6 98%View Details
136236-51-6 98%View Details
136236-51-6 -
 Rasagiline 95% CAS 136236-51-6View Details
Rasagiline 95% CAS 136236-51-6View Details
136236-51-6 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1