VIGABATRIN
Synonym(s):(±)-γ-Vinyl-GABA;(±)-4-Aminohexenoic acid;(±)-Vigabatrin;(R,S)-4-Amino-5-hexenoic acid
- CAS NO.:60643-86-9
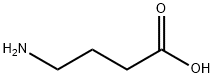
- Empirical Formula: C6H11NO2
- Molecular Weight: 129.16
- MDL number: MFCD00274577
- EINECS: 637-414-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-18 14:26:18
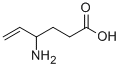
What is VIGABATRIN?
Description
Vigabatrin, the gamma-vinyl derivative of GABA, is a new anticonvulsant reportedly effective in the treatment of intractable seizures unresponsive to currently available therapy. Mechanistically vigabatrin is a potent irreversible GABA aminotransferase inhibitor which modifies the enzyme's active-site by Michael addition. Other potential indications have been suggested for vigabatrin, including depression and schizophrenia.
Chemical properties
White or almost white powder.
Originator
Merrell Dow (United Kingdom)
The Uses of VIGABATRIN
Vigabatrin is a selective GABA transaminase inhibitor.
The Uses of VIGABATRIN
antineoplastic
The Uses of VIGABATRIN
antibiotic
What are the applications of Application
Vigabatrin is a structural analog of GABA and a selective GABA transaminase inhibitor that does not bind to GABA receptors.
Definition
ChEBI: A gamma-amino acid having a gamma-vinyl GABA structure. It is an irreversible inhibitor of gamma-aminobutyric 664 acid transaminase
Manufacturing Process
Step A: 4-Formyloxy-3-hydroxy-1-butene
A solution of erythritol (50 g, 0.5 mole) in aqueous formic acid (150 g, 75%)
was heated above 100°C, 12 hours, then water and formic acid were distilled
off and the reaction mixture was heated above 200°C with a Bunsen burner.
The product was collected by distillation (b.p. 230°C, 30 g) and should be
rectified (b.p. 90°C, 15 mm).
Step B: Ethyl 6-formyloxy-4-hexanoate
A solution of 4-formyloxy-3-hydroxy-1-butene (1.06 g, 10 mmol) and
propionic acid (1 drop) in triethylorthoacetate (6 g, 40 mmol) was heated at
140°C under conditions for distillative removal of ethanol. After 2 hours, the
excess of ethylorthoacetate was removed by distillation in vacuo. The residue
was hydrolysed with water and extracted with AcOEt. The product was purified
by flash chromatography on SiO 2 (eluant AcOEt:hexane, 2:8) (1 g, 60%) but
distillative purification is preferred when larger quantities are involved.
Step C: Ethyl 6-hydroxy-4-hexanoate
A solution of 6-formyloxy-6-hexanoate (0.9 g, 5 mmol) in absolute EtOH (10
mL) containing few drops of a saturated solution of alcoholic HCl gas was left
2 hours at 20°C. The solvent was removed in vacuo and the residue was used
for the next step without further purification (0.7 g, quantitative). This
compound was found to be partially decomposed by flash chromatography on
SiO 2 .
Step D: Ethyl 4-trichloroacetamido-5-hexanoate
Sodium hydride (0.03 g of a 50% dispersion in oil, 0.5 mmol, was added to a
solution of ethyl 6-hydroxy-4-hexanoate (0.7 g, 5 mmol) and
trichloroacetonitrile (0.6 g, 5 mmol) in anhydrous ether (50 mL) under
N2at0°C. After 1hour, ethanol (0.5 mmol) was added and the solvent was
removed in vacuo. The formation of the imidate was controlled by NMR (NH,
about.8.5 ppm). A solution of the crude imidate in xylene (30 mL) was heated
at reflux 48 hours. Then the solvent was removed in vacuo and the residue
was purified by flash chromatography on SiO 2 (eluant AcOEt:hexane, 2:8) to give the title product (1.1 g, about 70%). A sample was distilled for analysis
(b.p. 150°C, 0.5 mm Hg).
Step E: 4-Amino-5-hexenoic acid
A suspension of ethyl 4-trichloroacetoamido-5-hexanoate (0.3 g, 1 mmol) in 6
N HCl (10 mL) was heated under reflux 6 hours. Then the mixture was
concentrated in vacuo, diluted with water (10 mL), washed twice with AcOEt,
and dried in uacuo to give the title product (0.18 g, 100%). NMR, TLC
(NH 4 OH:EtOH, 3:7) are identical with those of an authentic sample of 4-
amino-5-hexenoic acid
brand name
Sabril (Hoechst Marion Roussel);Sobril tab 25 mg.
Therapeutic Function
Antiepileptic
World Health Organization (WHO)
Vigabatrin, an irreversible inhibitor of GABA-transaminase was introduced in 1989 as a anticonvulsant for management of epilepsy unresponsive to other antiepilepsy agents. In 1991 it was refused registration in Norway because it induced toxic changes, including microvacuolation in the brain of two animal species, at doses that are close to therapeutic dosage levels in man. It is still marketed in Sweden and the United Kingdom.
Biological Functions
Vigabatrin (Sabril) is a relatively specific irreversible inhibitor
of GABA-transaminase (GABA-T), the major
enzyme responsible for the metabolism of GABA in the
mammalian CNS. As a result of inhibition of GABA-T,
there is an increase in the concentration of GABA in the
brain and consequently an increase in inhibitory neurotransmission.
Vigabatrin is well absorbed orally and is
distributed to all body systems.The major route of elimination
for vigabatrin is renal excretion of the parent compound;
no metabolites have been identified in humans.
At present, the primary indication for vigabatrin is
in the treatment of patients with partial seizures, but it
appears to be an effective and generally well tolerated
antiepileptic medication for other seizure types as well.
It should not be used in patients with absence epilepsy
or with myoclonic seizures. Vigabatrin is not approved
as an AED in the United States, although it is approved
in many other countries.
General Description
Vigabatrin, a 4-vinyl analog of GABA, produces its pharmacologicalaction by irreversibly blocking GABA catabolismcatalyzed by GABA-T as discussed earlier. It is marketedin Europe and Canada as an adjunctive treatment ofpatients with partial seizures, but it has yet to gain FDA approvalin the United States even after extensive clinical trials.The main concern with this drug is its ability to causea reversible visual field defect associated with retinal functionin the eyes.
Biological Activity
Selective GABA-T inhibitor. Anticonvulsant.
Clinical Use
Antiepileptic
Synthesis
The reaction of 1,4-dichloro-2- butene with diethyl malonate in the presence of sodium ethoxide as catalyst in refluxing ethanol gives 1,1-bis(ethoxycarbonyl)-2-vinylcyclopropane (8), which by reaction with gaseous ammonia in DMF is converted into 3-carboxamido- 5-vinyl-2-pyrrolidone (9). This compound is treated with HCl in refluxing acetic acid to yield vigabatrin.

Drug interactions
Potentially hazardous interactions with other drugs
Antidepressants: anticonvulsant effect antagonised,
convulsive threshold lowered; avoid with St John’s
wort.
Antiepileptics: concentration of phenytoin reduced.
Antimalarials: mefloquine antagonises
anticonvulsant effect.
Antipsychotics: anticonvulsant effect antagonised.
Orlistat: increased risk of convulsions.
Metabolism
Vigabatrin is not significantly metabolised. About
60-80
% of an oral dose is excreted in urine as unchanged
drug.
Properties of VIGABATRIN
| Melting point: | 209° |
| Flash point: | 9℃ |
| storage temp. | Desiccate at +4°C |
| solubility | Freely soluble in water, slightly soluble in methanol, practically insoluble in methylene chloride. |
| CAS DataBase Reference | 60643-86-9(CAS DataBase Reference) |
Safety information for VIGABATRIN
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H225:Flammable liquids H370:Specific target organ toxicity, single exposure |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P311:Call a POISON CENTER or doctor/physician. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for VIGABATRIN
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 VIGABATRIN 98%View Details
VIGABATRIN 98%View Details -
 Vigabatrin 98%View Details
Vigabatrin 98%View Details -
 68506-86-5 / 60643-86-9 Vigabatrin 98%View Details
68506-86-5 / 60643-86-9 Vigabatrin 98%View Details
68506-86-5 / 60643-86-9 -
 Vigabatrin 98%View Details
Vigabatrin 98%View Details
68506-86-5 / 60643-86-9 -
 68506-86-5 / 60643-86-9 Vigabatrin 99%View Details
68506-86-5 / 60643-86-9 Vigabatrin 99%View Details
68506-86-5 / 60643-86-9 -
 Vigabatrin >95% CAS 60643-86-9View Details
Vigabatrin >95% CAS 60643-86-9View Details
60643-86-9 -
 Vigabatrin solution CASView Details
Vigabatrin solution CASView Details -
 VigabatrinView Details
VigabatrinView Details
60643-86-9
