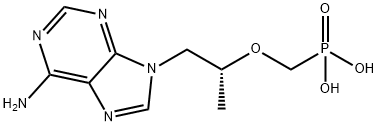
Tenofovir
Synonym(s):(R)-9-(2-Phosphonomethoxypropyl)adenine;(R)-PMPA;[[(1R)-2-(6-Amino-9H-purin-9-yl)-1-methylethoxy]methyl]phosphonic acid monohydrate;1-(6-Aminopurin-9-yl) propan-2-yl-oxymethylphosphonic acid;TEN
- CAS NO.:147127-20-6
- Empirical Formula: C9H14N5O4P
- Molecular Weight: 287.21
- MDL number: MFCD07357269
- EINECS: 604-571-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 18:10:20
What is Tenofovir?
Description
Tenofovir is an analog of adenosine monophosphate that has antiviral activity. It is converted by cellular enzymes to tenofovir diphosphate, an obligate chain terminator that inhibits the activity of HIV reverse transcriptase and hepatitis B virus polymerase. Tenofovir diphosphate is a weak inhibitor of mammalian DNA polymerases α and β and mitochondrial DNA polymerase γ. For in vivo and cell culture use, tenofovir is supplied as a water soluble prodrug in the form of tenofovir disoproxil (fumarate) , which increases the intracellular diphosphorylated compound >1,000-
Chemical properties
White Crystalline Solid
The Uses of Tenofovir
Tenofovir is an acyclic phosphonate nucleotide analogue and reverse transcriptase inhibitor. It inhibits the activity of HIV reverse transcriptase through competing with the natural substrate deoxyadenosine 5’-triphosphate, causing the termination of DNA chain. It is used for the treatment of chronic heptatitis B as well as prevention and treatment of HIV/AIDS.
Definition
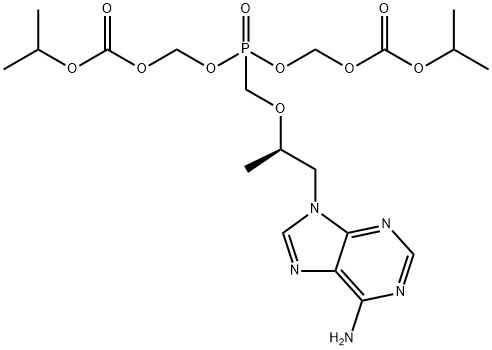
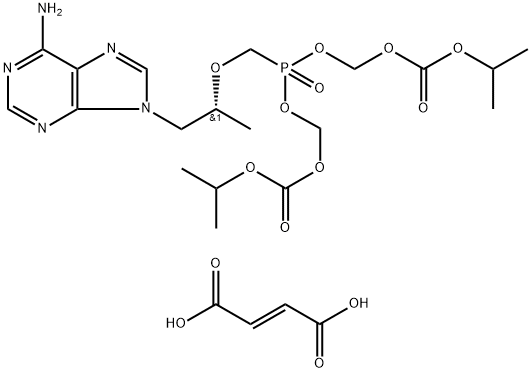
ChEBI: A member of the class of phosphonic acids that is methylphosphonic acid in which one of the methyl hydrogens is replaced by a [(2R)-1-(6-amino-9H-purin-9-yl)propan-2-yl]oxy group. An inhibitor of HIV-1 reverse transcriptase, the bis(isopropyloxycarbonyloxy ethyl) ester (disoproxil ester) prodrug is used as the fumaric acid salt in combination therapy for the treatment of HIV infection.
Indications
Tenofovir disoproxil fumarate (Viread) is a prodrug of tenofovir, a phosphorylated adenosine nucleoside analogue, and is the only available agent of its class. It is converted by cellular enzymes to tenofovir diphosphate, which competes with deoxyadenosine triphosphate (dATP) for access to reverse transcriptase and causes chain termination following its incorporation. Tenofovir was approved as part of a combination therapy for HIV in adults who failed treatment with other regimens; it appears to be effective against HIV strains that are resistant to NRTIs.
Acquired resistance
HIV variants with the K65R mutation and the K70E mutation in the reverse transcriptase demonstrate reduced susceptibility to tenofovir.
Pharmaceutical Applications
A nucleotide analog structurally similar to adefovir.
EC50 values for HBV, assessed in the HepG2 2.2.15 cell
line, ranged from 0.14 to 1.5 μm; the cytotoxic concentration
exceeded 100 μm. A decline in HBV DNA levels below
105 copies/mL at 48 weeks of therapy in 100% of patients
receiving tenofovir compared with 44% on adefovir therapy
has been reported. There are also case reports of patients with
primary resistance to adefovir responding to tenofovir.
It is generally well tolerated in patients with chronic HBV;
the most common side effects include nausea and gastrointestinal
upset, headache, dizziness, fatigue and rash.
Biochem/physiol Actions
Tenofovir has a low oral bioavailability. Hence, it is available as a prodrug called tenofovir disoproxil fumarate. Once ingested, tenofovir disoproxil fumarate is hydrolyzed to tenofovir and phosphorylated. This is then incorporated into the viral DNA which leads to chain termination. Tenofovir is also effective against hepatitis B virus.
Clinical Use
Treatment of HIV infection in adults and children (in combination with other antiretroviral drugs)
Side Effects
Tenofovir is taken once daily and is generally well tolerated, perhaps because it produces less mitochondrial toxicity than the NRTIs. Nausea, vomiting, flatulence, and diarrhea occur in 10% or fewer patients. Resistance to tenofovir has been documented, and cross-resistance to NRTIs may occur.
Storage
Store at -20°C
Properties of Tenofovir
| Melting point: | 276-280°C |
| Boiling point: | 616.1±65.0 °C(Predicted) |
| alpha | D +21° (c = 1 in 0.1M HCl) |
| Density | 1.79±0.1 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | Aqueous Acid (Sparingly), DMSO (Slightly, Heated), Water (Slightly, Heated) |
| form | powder |
| pka | 2.36±0.10(Predicted) |
| color | white to beige |
| optical activity | [α]/D -20 to -26°, c = 0.5 in 1 M HCl |
| Water Solubility | 13.4 mg/mL (25 ºC) |
| Merck | 14,9146 |
| InChI | InChI=1S/C9H14N5O4P/c1-6(18-5-19(15,16)17)2-14-4-13-7-8(10)11-3-12-9(7)14/h3-4,6H,2,5H2,1H3,(H2,10,11,12)(H2,15,16,17)/t6-/m1/s1 |
| CAS DataBase Reference | 147127-20-6(CAS DataBase Reference) |
Safety information for Tenofovir
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H332:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Tenofovir
| InChIKey | SGOIRFVFHAKUTI-ZCFIWIBFSA-N |
| SMILES | P(CO[C@H](C)CN1C2C(N=C1)=C(N)N=CN=2)(=O)(O)O |
Tenofovir manufacturer
TIKVAH PHARMA SOLUTIONS PVT LTD
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
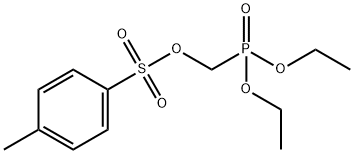
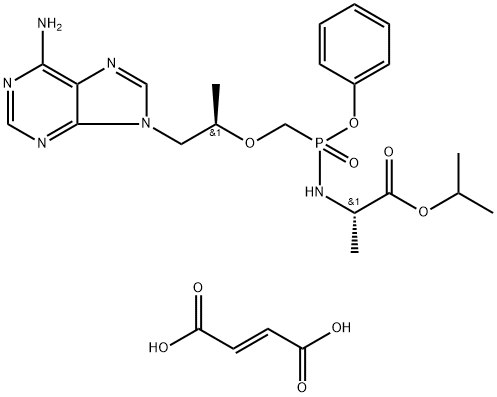
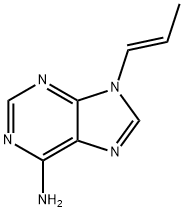

![9-[(R)-2-(Phosphonomethoxy)propyl]adenine monohydrate](https://img.chemicalbook.in/CAS/GIF/206184-49-8.gif)
You may like
-
 147127-20-6 98%View Details
147127-20-6 98%View Details
147127-20-6 -
 147127-20-6 (R)-(1-(6-amino-9H-purin-9-yl)propan-2-yloxy)methylphosphonic acid hydrate 98%View Details
147127-20-6 (R)-(1-(6-amino-9H-purin-9-yl)propan-2-yloxy)methylphosphonic acid hydrate 98%View Details
147127-20-6 -
 Tenofovir 99%View Details
Tenofovir 99%View Details -
 Tenofovir 98%View Details
Tenofovir 98%View Details -
 147127-20-6 Tenofovir 98%View Details
147127-20-6 Tenofovir 98%View Details
147127-20-6 -
 Tenofovir 97% CAS 147127-20-6View Details
Tenofovir 97% CAS 147127-20-6View Details
147127-20-6 -
 Tenofovir CAS 147127-20-6View Details
Tenofovir CAS 147127-20-6View Details
147127-20-6 -
 Tenofovir CAS 147127-20-6View Details
Tenofovir CAS 147127-20-6View Details
147127-20-6
