Lanreotide
- CAS NO.:108736-35-2
- Empirical Formula: C54H69N11O10S2
- Molecular Weight: 1096.32
- MDL number: MFCD03424071
- EINECS: 689-178-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-26 17:28:21
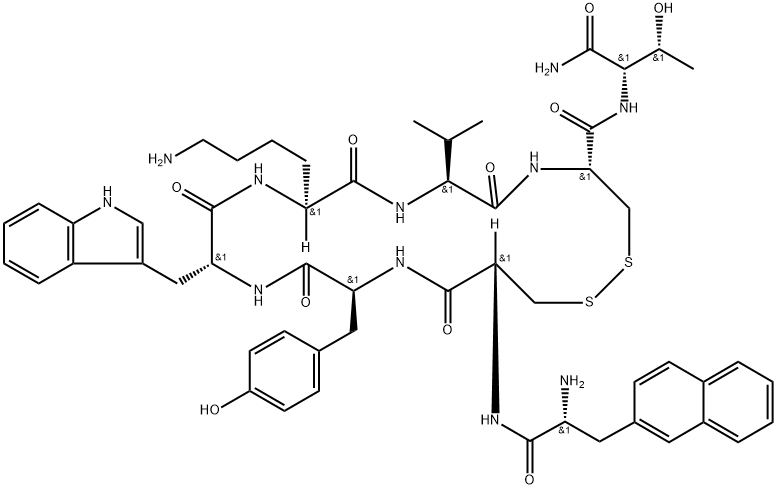
What is Lanreotide?
Absorption
Lanreotide forms a drug depot at the site of injection; therefore, there are 2 phases that describe the absorption of Lanreotide: 1. Initial rapid subcutaneous release during the first few days of treatment where drug that has not precipitated is rapidly absorbed. 2. Slow release of drug from the depot via passive diffusion. Absorption is independent of body weight, gender, and dosage.
Toxicity
The most common adverse events are GI related, occurring in 67-84% of patients, and are typically mild to moderate. GI related effects are often transient, improve with subsequent injections, and most frequently include diarrhea and abdominal pain. Other GI symptoms such as nausea, vomiting, and abdominal distension are less common. It is not clear whether or not GI effects are dose related. Adverse effects relating to site of injection occur in 43% of patients and are more common in patients who self-inject as opposed to those who had health-care professionals administer the injection. A small number of patients report newly impaired glucose tolerance, fasting glucose or diabetes mellitus. Patients being treated for diabetes mellitus may experience hypoglycemia. After 1 year, up to 30% of patients may experience gallstone formation and the presence of sludge within the gallbladder due to inhibition of gallbladder and GI motility. This may be influenced by previous exposure to somatostatin analogues. Other adverse effects include reduction in left ventricular end-diastolic and end-systolic volumes, bradycardia, nasopharyngitis, and alopecia. Lanreotide is classified as Pregnancy Category C.
Chemical properties
White to off-white lyophilised powder
The Uses of Lanreotide
Antineoplas tic.
Indications
Lanreotide is indicated for the long-term treatment of patients with acromegaly who have had an inadequate response to, or cannot be treated with, surgery and/or radiotherapy. It is also indicated in the treatment of adult patients with unresectable, well- or moderately-differentiated, locally advanced or metastatic gastroenteropancreatic neuroendocrine tumors (GEP-NETs) to improve progression-free survival.
Lanreotide is additionally indicated for the treatment of adults with carcinoid syndrome - when used, it reduces the frequency of short-acting somatostatin analog rescue therapy.
Background
Lanreotide is a drug employed in the management of acromegaly (a hormonal condition caused by excess growth hormone) in addition to symptoms caused by neuroendocrine tumors, especially carcinoid syndrome. This drug is a long-acting analog of the drug somatostatin, a growth hormone inhibitor. Lanreotide is manufactured by the company, Ipsen Pharmaceuticals as lanreotide acetate, and marketed as Somatuline. It is approved in several countries worldwide, including the United Kingdom, Australia, and Canada. Lanreotide was first approved for use in the United States by the FDA on August 30, 2007.
Pharmacokinetics
Lanreotide exhibits antisecretory effects through cAMP suppression, and activation of ion currents such as K+ and Ca2+ which leads to hyperpolarization of the membrane and inhibition of Ca2+ mediated depolarization. Furthermore, through direct and indirect mechanisms, Lanreotide has potent antiproliferative effects.
Metabolism
Not Available
Properties of Lanreotide
| Boiling point: | 1508.2±65.0 °C(Predicted) |
| Density | 1.40±0.1 g/cm3(Predicted) |
| storage temp. | Keep in dark place,Sealed in dry,Store in freezer, under -20°C |
| pka | 9.90±0.15(Predicted) |
Safety information for Lanreotide
| Signal word | Warning |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H361:Reproductive toxicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Lanreotide
Lanreotide manufacturer
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 108736-35-2 Lanreotide 98%View Details
108736-35-2 Lanreotide 98%View Details
108736-35-2 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8