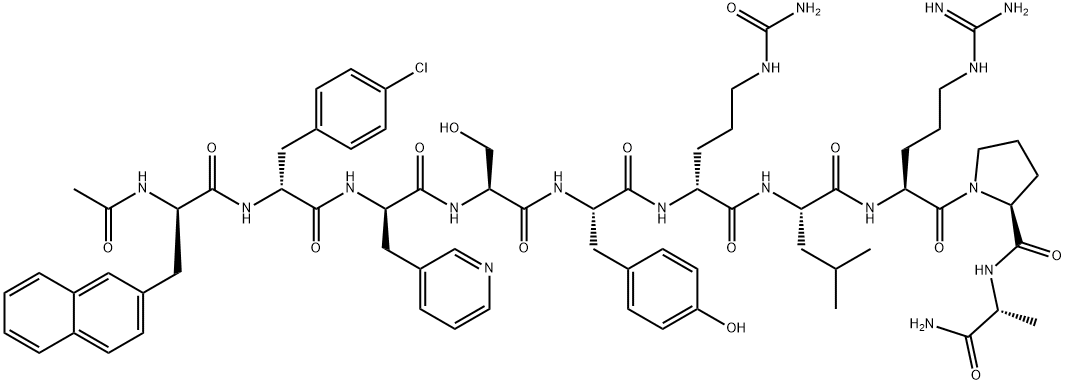
Cetrorelix
Synonym(s):Cetrotide;N-acetyl-3-(2-naphthalenyl)-D-alanyl-4-chloro-D-phenylalanyl-3-(3-pyridinyl)-D-alanyl-L-seryl-L-tyrosyl-N5-(aminocarbonyl)-D-ornithyl-L-leucyl-L-arginyl-L-prolyl-D-alaninamide acetic acid salt;NS-75A;SB-75
- CAS NO.:120287-85-6
- Empirical Formula: C70H92ClN17O14
- Molecular Weight: 1431.04
- MDL number: MFCD00884467
- EINECS: 686-384-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-09 15:48:30
What is Cetrorelix?
Absorption
Rapidly absorbed following subcutaneous injection. The mean absolute bioavailability following subcutaneous administration to healthy female subjects is 85%.
Description
Cetrorelix was launched in Germany for the treatment of female infertility. It is a decapeptidic analog of luteinizing hormone-releasing hormone (LH-RH) bearing structural modifications in the crucial positions 1, 2, 3, 6 and 10 : [Ac-D-Nal1, D-4-CI Phe-2, D-Pal3, D-Cit6, D-Alal0]-GnRH. Cetrorelix is an extremely potent and long acting gonadotrophin releasing hormone (GnRH) antagonist and thus blocks gonadotrophins and sex steroid secretion immediately after administration. Moreover, it has a low histamine-releasing potency. Cetrorelix will be the first LH-RH antagonist approved worldwide. In several Phase III clinical trials, female patients receiving Cetrorelix had a successful controlled ovulation thus avoiding a premature LH-surge. Cetrorelix could be a first-choice in-vitro fertilization (IVF) treatment without the complications of current controlled ovarian hyperstimulation protocols. Cetrorelix is currently under clinical investigation for the treatment of diverse sex hormone dependent disorders such as benign prostate hypertrophy, breast, ovarian or prostate cancers and diverse gynaecological disorders.
Originator
Asta Medica (Germany)
The Uses of Cetrorelix
Cetrorelix Acetate is an gonadotropin-releasing hormone antagonist and is used to treat hormone-sensitive cancers of the prostate and breast.
Indications
For the inhibition of premature LH surges in women undergoing controlled ovarian stimulation
Background
Cetrorelix is a man-made hormone that blocks the effects of Gonadotropin Releasing Hormone (GnRH). GnRH controls another hormone that is called luteinizing hormone (LH), which is the hormone that starts ovulation during the menstrual cycle. When undergoing hormone treatment sometimes premature ovulation can occur, leading to eggs that are not ready for fertilization to be released. Cetrorelix does not allow the premature release of these eggs to occur.
Definition
ChEBI: A synthetic ten-membered oligopeptide comprising N-acetyl-3-(naphthalen-2-yl)-D-alanyl, 4-chloro-D-phenylalanyl, 3-(pyridin-3-yl)-D-alanyl, L-seryl, L-t rosyl, N5-carbamoyl-D-ornithyl, L-leucyl, L-arginyl, L-prolyl, and D-alaninamide residues coupled in sequence. A gonadotrophin-releasing hormone (GnRH antagonist, it is used for treatment of infertility and of hormone-sensitive cancers of the prostate and breast.
brand name
Cetrotide (Serono).
Pharmacokinetics
Cetrorelix is a synthetic decapeptide with gonadotropin-releasing hormone (GnRH) antagonistic activity. GnRH induces the production and release of luteinizing hormone (LH) and follicle stimulating hormone (FSH) from the gonadotrophic cells of the anterior pituitary. Due to a positive estradiol (E2) feedback at midcycle, GnRH liberation is enhanced resulting in an LH-surge. This LH-surge induces the ovulation of the dominant follicle, resumption of oocyte meiosis and subsequently luteinization as indicated by rising progesterone levels. Cetrorelix competes with natural GnRH for binding to membrane receptors on pituitary cells and thus controls the release of LH and FSH in a dose-dependent manner.
Metabolism
In in vitro studies, cetrorelix was stable against phase I- and phase II-metabolism. Cetrorelix was transformed by peptidases, and the (1-4) peptide was the predominant metabolite.
Properties of Cetrorelix
| Melting point: | >259°C (dec.) |
| Density | 1.42±0.1 g/cm3(Predicted) |
| storage temp. | Refrigerator |
| solubility | DMSO (Slightly, Heated), Methanol (Very Slightly) |
| form | Solid |
| pka | 9.82±0.15(Predicted) |
| color | White to Off-White |
Safety information for Cetrorelix
Computed Descriptors for Cetrorelix
| InChIKey | SBNPWPIBESPSIF-MHWMIDJBSA-N |
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran



You may like
-
 Cetrorelix Acetate 98%View Details
Cetrorelix Acetate 98%View Details -
 120287-85-6 98%View Details
120287-85-6 98%View Details
120287-85-6 -
 Cetrorelix 98%View Details
Cetrorelix 98%View Details
120287-85-6 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
