SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H361:Reproductive toxicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. |
COMPUTED DESCRIPTORS
| Molecular Weight | 1096.3 g/mol |
|---|---|
| XLogP3 | 2.5 |
| Hydrogen Bond Donor Count | 13 |
| Hydrogen Bond Acceptor Count | 14 |
| Rotatable Bond Count | 17 |
| Exact Mass | 1095.46702979 g/mol |
| Monoisotopic Mass | 1095.46702979 g/mol |
| Topological Polar Surface Area | 406 Ų |
| Heavy Atom Count | 77 |
| Formal Charge | 0 |
| Complexity | 2000 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 9 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
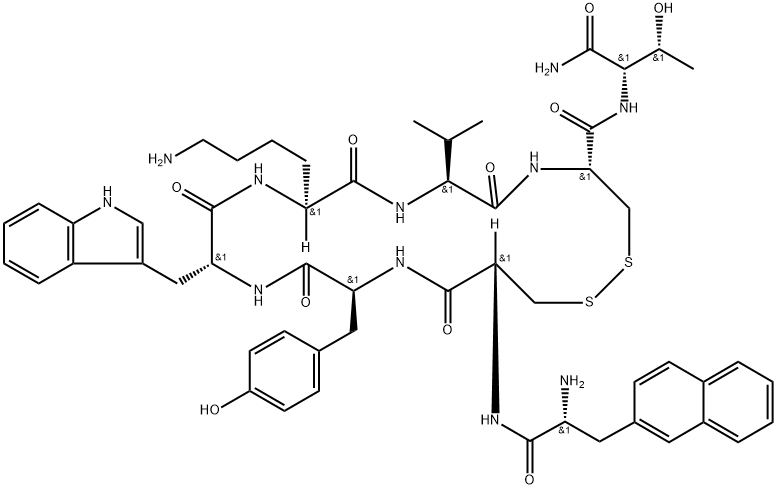
PRODUCT INTRODUCTION
description
Lanreotide is a drug employed in the management of acromegaly (a hormonal condition caused by excess growth hormone) in addition to symptoms caused by neuroendocrine tumors, especially carcinoid syndrome. This drug is a long-acting analog of the drug somatostatin, a growth hormone inhibitor. Lanreotide is manufactured by the company, Ipsen Pharmaceuticals as lanreotide acetate, and marketed as Somatuline. It is approved in several countries worldwide, including the United Kingdom, Australia, and Canada. Lanreotide was first approved for use in the United States by the FDA on August 30, 2007.