Liraglutide
- CAS NO.:204656-20-2
- Empirical Formula: C172H265N43O51
- Molecular Weight: 3751.202
- MDL number: MFCD31689263
- EINECS: 810-818-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-03-27 06:45:46
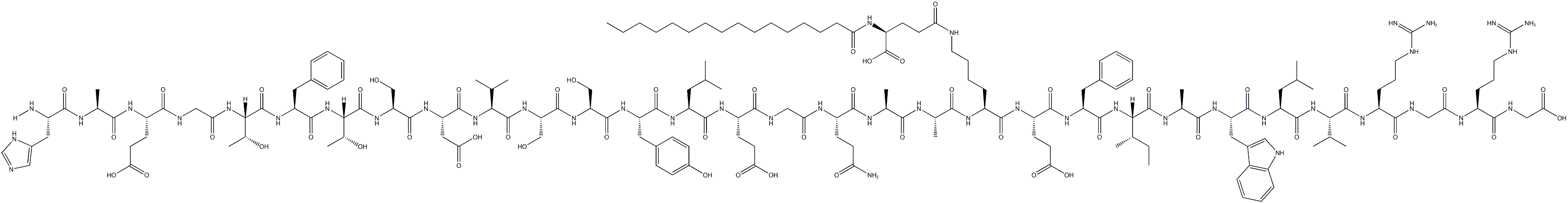
What is Liraglutide?
Chemical properties
Liraglutide is characterized as a white to almost white powder. It is freely soluble in aqueous base solutions (> 270 mg/mL), but its water solubility decreases below pH 7 and reaches its lowest level at pH 4-5 (approximately 0.05 mg/mL). Solubility increases marginally at pH 2.5 where it is very slightly soluble (≤ 0.8 mg/mL). Liraglutide is soluble in methanol (68 mg/mL) and very slightly soluble in ethanol (1.1 mg/mL). The isoelectric point of liraglutide is approximately 4.9. The pH of a 1 mg/mL aqueous solution of drug substance is approximately 9.3.
The Uses of Liraglutide
Liraglutide is a new type of hypoglycemic drug that activates AMP-activated protein kinase thus enhancing insulin sensitivity. It not only has a significant hypoglycemic effect, but also has the functions of weight loss, blood pressure reduction, and improvement of blood lipid profile.
Liraglutide, a human glucagon-like peptide-1 (GLP-1) receptor agonist, slows gastric emptying, increases satiety, and reduces patient eating, while reducing the brain's desire to eat and allowing the body to Increased energy expenditure plays a role in weight loss; it prevents the liver from producing too much glucose and promotes the pancreas to produce more insulin to lower blood sugar; in addition, liraglutide also has cardiovascular benefits and kidney protection.
Definition
ChEBI: Liraglutide, a lipopeptide and polypeptide, is an analogue of human GLP-1 in which the lysine residue at position 27 is replaced by arginine and a hexadecanoyl group attached to the remaining lysine via a glutamic acid spacer. It is used alongside diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. Liraglutide functions as a glucagon-like peptide-1 receptor agonist and serves as a neuroprotective agent.
General Description
Liraglutide is GLP-1(1–37) modified at Ly: the -amino group is acylated with an (N-hexadecanoyl)glutam- -yl moiety that noncovalently but avidly binds tohuman serum albumin. (Also, Lys is conservatively replacedwith Arg.) These changes provide for a greatly extendedserum t1?2 of~10 to 15 hours after sc administration(0.6–1.8 mg), allowing for once-daily dosing.Liraglutide (Victoza, Novo Nordisk) is awaiting approvalby the FDA, EMEA, and the Koˉ roˉ -shoˉ (Japan).
Clinical Use
Glucogen-like peptide-1 analogue:
Treatment of type 2 diabetes mellitus in combination
with other antidiabetic therapy
Side Effects
The most frequent adverse events associated with liraglutide therapy were gastrointestinal in nature, in accordance with GLP-1 receptor activation, and included nausea, diarrhea, vomiting, constipation, abdominal pain, and dyspepsia.
Clinical claims and research
Liraglutide, the GLP-1 receptor agonist to reach the market, possesses a 97% homology to GLP-1 with only two amino acid changes and the addition of a fatty acid side chain. Specifically, the lysine in position 34 has been replaced with an arginine, and the lysine in position 26 has been modified with a C16 acyl chain via a glutamoyl spacer. Liraglutide derives its resistance to DPP-4 degradation from its propensity to form micelles and to bind to albumin. Unlike its predecessor exenatide, which requires two daily subcutaneous injections before the first and last meals of the day, liraglutide is approved as a once-daily treatment regimen and may be used in combination with metformin or a sulfonylurea in patients with insufficient glycemic control with either monotherapy or combined dual therapy. It is also approved in combination with the dual therapy of metformin and a thiazolidinedione in patients with insufficient glycemic control. Liraglutide displayed a binding potency of 61 pM (EC50= 55 pM for GLP-1) for the cloned human GLP-1 receptor.
Mode of action
Liraglutide is an acylated human glucagon-like peptide-1 (GLP-1) receptor agonist with 97% amino acid sequence homology to endogenous human GLP-1(7-37). Like endogenous GLP-1, liraglutide binds to and activates the GLP-1 receptor, a cellsurface receptor coupled to adenylyl cyclase activation through the stimulatory G-protein, Gs. Endogenous GLP-1 has a half-life of 1.5-2 minutes due to degradation by the ubiquitous endogenous enzymes, dipeptidyl peptidase 4 (DPP-4) and neutral endopeptidases (NEP). Unlike native GLP-1, liraglutide is stable against metabolic degradation by both peptidases and has a plasma half-life of 13 hours after subcutaneous administration.
Properties of Liraglutide
| Melting point: | >182°C (dec.) |
| storage temp. | Refrigerator |
| solubility | DMSO (Slightly), Water (Slightly) |
| form | Solid |
| color | White to Off-White |
Safety information for Liraglutide
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Liraglutide
Liraglutide manufacturer
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran




You may like
-
 204656-20-2 Liraglutide 98%View Details
204656-20-2 Liraglutide 98%View Details
204656-20-2 -
 Liraglutide 98%View Details
Liraglutide 98%View Details -
 Liraglutide 99%View Details
Liraglutide 99%View Details
204656-20-2 -
 Liraglutide 204656-20-2 99%View Details
Liraglutide 204656-20-2 99%View Details
204656-20-2 -
 204656-20-2 Liraglutide 98%View Details
204656-20-2 Liraglutide 98%View Details
204656-20-2 -
 Liraglutide 98%View Details
Liraglutide 98%View Details
204656-20-2 -
 Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
67967-07-1 -
 132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6
