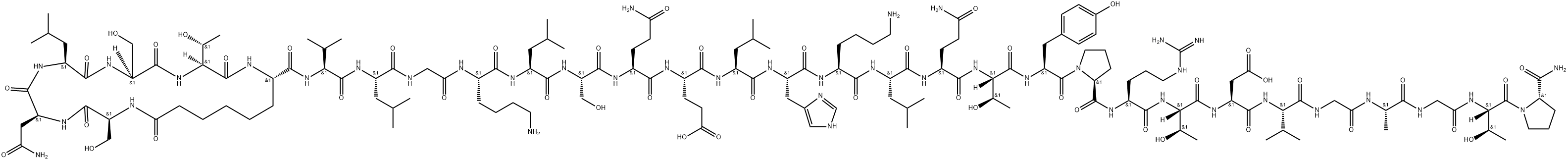
Degarelix
Synonym(s):Ac-D2Nal-D4Cpa-D3Pal-Ser-4Aph(L-hydroorotyl)-D4Aph(carbamoyl)-Leu-ILys-Pro-DAla-NH2 acetate salt;ASP3550 acetate;FE 200486;FE200486;
- CAS NO.:214766-78-6
- Empirical Formula: C82H103ClN18O16
- Molecular Weight: 1632.26
- MDL number: MFCD05860888
- EINECS: 807-277-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-20 20:09:13
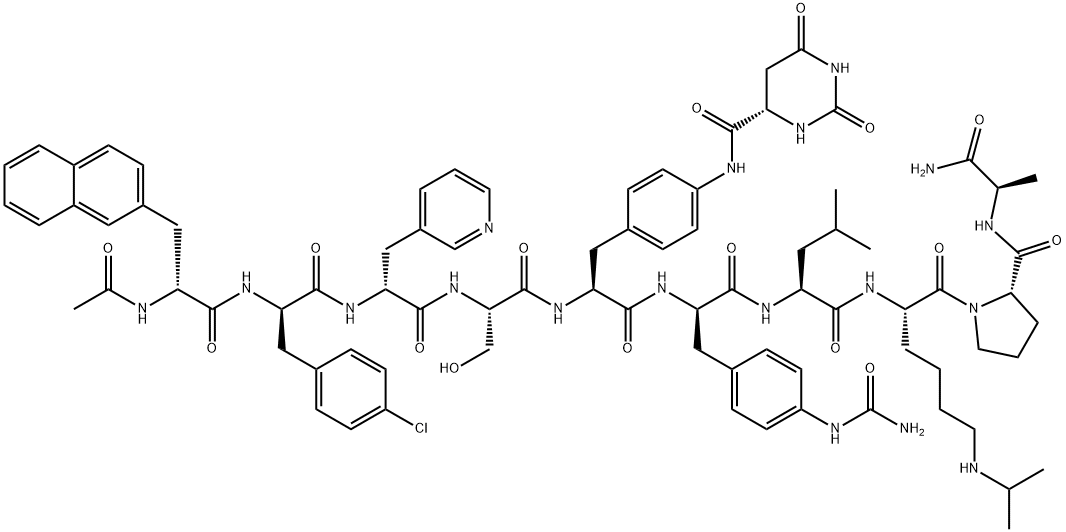
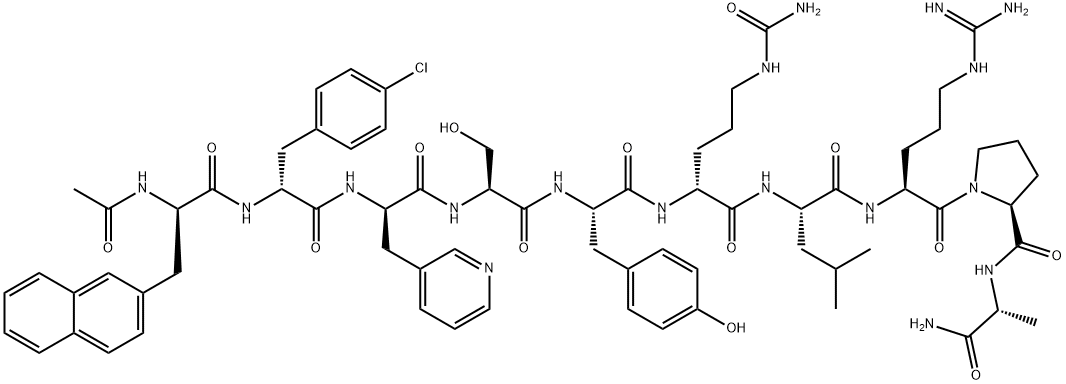
What is Degarelix ?
Absorption
Degarelix forms a depot at the site of injection after subcutaneous administration from which the drug slowly released into circulation. After a single bolus dose of 2mg/kg, peak plasma concentrations of degarelix occured within 6 hours at a concentration of 330 ng/mL. Ki = 0.082 ng/mL and 93% of receptors were fully suppressed; MRT = 4.5 days.
Toxicity
The most commonly observed adverse reactions (> 10%) during degarelix therapy included injection site reactions (e.g., pain, erythema, swelling, or induration), hot flashes, increased weight, and increases in serum levels of transaminases and gamma-glutamyltransferase (GGT).
Description
Antagonists of GnRH have proven to be an effective therapy for hormonally regulated cancers, such as prostate and some types of breast. As analogs of GnRH, they bind competitively and reversibly to GnRH receptors in the pituitary gland, thereby blocking the release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH). In men, the reduction of LH triggers the ablation of testosterone secretion from the testes, and these castration-like levels have been essential in the effective management of advanced prostate cancer. In comparison to GnRH agonists, antagonists do not suffer from a potential flare of the disease as a result of an initial stimulation of the hypothalamic-pituitary-gonadal axis prior to down-regulation of the GnRH receptor. Moreover, GnRH antagonists provide beneficial effects more rapidly postdosing and result in a more efficient suppression of gonadotropin levels. With this in mind, degarelix acetate has been launched as a third-generation GnRH antagonist for the treatment of prostate cancer, and it joins other third-generation agents, ganirelix and cetronelix, on the market.
Originator
Ferring Pharmaceutical (Switzerland)
The Uses of Degarelix
Degarelix, is a competitive and reversible gonadotropin-releasing hormone receptor (GnRHR) antagonist.
The Uses of Degarelix
Advanced hormone-dependent prostate carcinoma
Background
Degarelix is used for the treatment of advanced prostate cancer. Degarelix is a synthetic peptide derivative drug which binds to gonadotropin-releasing hormone (GnRH) receptors in the pituitary gland and blocks interaction with GnRH. This antagonism reduces luteinising hormone (LH) and follicle-stimulating hormone (FSH) which ultimately causes testosterone suppression. Reduction in testosterone is important in treating men with advanced prostate cancer. Chemically, it is a synthetic linear decapeptide amide with seven unnatural amino acids, five of which are D-amino acids. FDA approved on December 24, 2008.
Indications
Degaralix is used for the management of advanced prostate cancer.
Definition
ChEBI: Degarelix is a polypeptide.
brand name
Firmagon
Pharmacokinetics
Degarelix is a synthetic derivative of GnRH decapeptide, the ligand of the GnRH receptor. Gonadotropin and androgen production result from the binding of endogenous GnRH to the GnRH receptor. Degarelix antagonizes the GnRH receptor which in turn blocks the release of LH and FSH from the pituitary. LF and FSH decreases in a concentration-dependent manner. The reduction in LH leads to a decrease in testosterone release from the testes.
Clinical Use
Ferring launched degarelix acetate, a gonadotrophin-releasing hormone (GnRH) antagonist, in 2009 in the U.S. for the treatment of prostate cancer. The compound has been approved by the E.U. for the same indication, and in the same year it was launched in the UK and Germany. Degarelix has been developed as a one-month or three-month sustained-release injectable formulation. Compared to other GnRH antagonists, degarelix displays improved aqueous solubility, longer acting effects and weaker histamine-releasing properties.
Side Effects
The most common adverse events included injection site reactions (pain, erythema, swelling, or induration), hot flashes, increased weight, and increases in serum levels of transaminases and gamma-glutamyltransferase. In addition to being contraindicated in patients with a previous hypersensitivity to degarelix, it should not be administered to women who are or may become pregnant as fetal harm can occur. Since long-term androgen deprivation therapy prolongs the QT interval, physicians should consider whether the benefits of degarelix outweigh the potential risks in patients with congenital long QT syndrome, electrolyte abnormalities, or congestive heart failure or in patients taking antiarrhythmic medications.
Synthesis
The synthesis of degarelix acetate employed iterative peptide coupling and protection/de-protection sequences in high yields (85¨C99%), and this sequence is described in the scheme. Boc-D-alanine (21) was immobilized via MBHA resin (Bachem) by reaction with diisopropyl carbodiimide (DIC) and 1-hydroxybenzotriazole (HOBT). The resulting product was treated with trifluoroacetic acid (TFA) to remove the N-Boc protecting group to reveal amine 22. The N-terminus of 22 was then subjected to sequential coupling and de-protection cycles with the following protected amino acids: N-Boc-L-proline, N-a- Boc-N6-isopropyl-N6-carbobenzoxy-L-lysine and N-Boc-L-leucine to give 23 and 24, respectively. The N-terminus of 24 was coupled with N-a-Boc-D-4-(Fmoc-amino)phenylalanine, followed by removal of the Fmoc group with piperidine in DMF to give the corresponding free aniline. The free aniline resin was then reacted with t-butyl isocyanate to generate the corresponding t-butyl urea followed by reaction with TFA to remove the Boc group to give the t-butyl urea amine 25. The N-terminus of 25 was coupled with N-a-Boc-L-4-(Fmoc-amino)phenylalanine, followed by removal of the Fmoc group with piperidine in DMF to generate the corresponding free aniline. The free aniline was reacted with L-hydroorotic acid, followed by reaction with TFA to liberate amine 26. Amine 26 was then coupled with O-benzylated-N-Boc-serine, followed by removal of the Boc group with TFA and reacting the resulting amine with N-a-Boc-D-(3-pyridyl)alanine and subsequent removal of the Boc group with TFA gave amine 27. Amine 27 was coupled with N-Boc-D-(4-chlorophenyl)alanine, followed by removal of the Boc group with TFA, and the resulting amine was then coupled with N-Boc-D-(2-naphthyl)alanine, followed by removal of its Boc group with TFA to give 28. Acylation of 28 with acetic anhydride followed by sequential treatment with HF and TFA resulted in cleavage from the resin, removal of the O-benzyl group, and conversion of the t-butyl urea to the corresponding NH2-urea, resulting in free degarelix. Finally, treatment with acetic acid provided degarelix acetate (V).
Metabolism
70% - 80% of degarelix is subject to peptide hydrolysis during its passage through the hepatobiliary system and then fecally eliminated. No active or inactive metabolites or involvement of CYP450 isozymes.
Metabolism
Undergoes peptide hydrolysis in the hepato-biliary system, and is mainly (70-80%) excreted as peptide fragments in the faeces.
Properties of Degarelix
| Density | 1.325±0.06 g/cm3(Predicted) |
| storage temp. | Keep in dark place,Inert atmosphere,Store in freezer, under -20°C |
| solubility | DMSO:10.0(Max Conc. mg/mL);6.13(Max Conc. mM) H2O:25.0(Max Conc. mg/mL);15.32(Max Conc. mM) |
| pka | 10.38±0.40(Predicted) |
| form | Solid |
| color | White to off-white |
Safety information for Degarelix
Computed Descriptors for Degarelix
| InChIKey | MEUCPCLKGZSHTA-XYAYPHGZSA-N |
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 214766-78-6 Degarelix Acetate 98%View Details
214766-78-6 Degarelix Acetate 98%View Details
214766-78-6 -
 Degarelix Acetate 99%View Details
Degarelix Acetate 99%View Details -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
