KP 103
Synonym(s):(αR,βR)-, α-(2,4-Difluorophenyl)-β-methyl-4-methylene-α-(1H-1,2,4-triazol-1-ylmethyl)-1-piperidineethanol;(2R,3R)-2-(2,4-Difluorophenyl)-3-(4-methylenepiperidin-1-yl)-1-(1H-1,2,4-triazol-1-yl)butan-2-ol;KP-103
- CAS NO.:164650-44-6
- Empirical Formula: C18H22F2N4O
- Molecular Weight: 348.39
- MDL number: MFCD00936406
- EINECS: 813-597-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
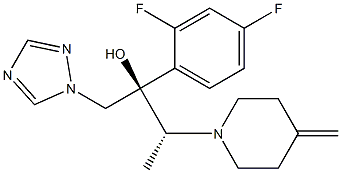
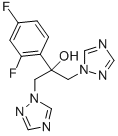
What is KP 103?
Description
In October 2013, efinaconazole (also known as KP-103) was approved in Canada as a 10% topical solution for the treatment of onychomycosis. Like other azole antifungal agents, efinaconazole acts by disrupting fungal cell membranes through inhibition of sterol 14α-demethylase, an enzyme involved in the biosynthesis of ergosterol, which is a key component of the fungal cell membrane. Efinaconazole has potent antifungal activity against clinical isolates of dermatophytes, including Trichophyton mentagrophyes (MIC80 =0.125 μg/mL) and Trichophyton rubrum (MIC80 =0.25 μg/mL), as well as against Candida and Malassezia species. Unlike other antifungal agents, efinaconazole retains activity in the presence of keratin, indicating that more unbound drug is available at the site of action. Efinaconazole is efficacious in guinea-pig models of fungal infection. Efinaconazole is prepared by reaction of an epoxide intermediate with 4-methylenepiperidine.
Description
Efinaconazole is a broad-spectrum triazole antifungal agent with activity against Acremonium, Aspergillus, Candida, Cryptococcus, Epidermophyton, Fusarium, Microsporum, Paecilomyces, Pseudallescheria, Scopulariopsis, Trichophyton, and Trichosporon. It inhibits the growth of T. rubrum and T. mentagrophytes clinical isolates with MIC values ranging from ≤2.0 to 60 ng/ml and of C. albicans isolates with MIC values ranging from ≤0.5 to >250 ng/ml. Efinaconazole inhibits sterol 14α-demethylase, which arrests ergosterol biosynthesis at the fungal membrane. It inhibits ergosterol biosynthesis in T. mentagrophytes and C. albicans with IC50 values of 7.0 and 0.40 ng/ml, respectfully. Topical formulations containing efinaconazole have been used for the treatment of onychomycosis.
Originator
Kaken Pharmaceuticals (Japan)
The Uses of KP 103
Efinaconazole has been used as:
- a topical anti-onychomycosis drug to determine its effects on Trichophyton rubrum and Trichophyton interdigitale
- as an anti-fungal agent to study its permeability into the nail lysates
- as an anti-fungal agent to study its effects on Candida africana and Candida dubliniensis
Indications
Indicated in the treatment of fungal infection of the nail, known as onychomycosis.
Background
Efinaconazole is a 14 alpha-demethylase inhibitor indicated in the treatment of fungal infection of the nail, known as onychomycosis. It was approved for use in Canada and the USA in 2014 and is marketed by Valeant Pharmaceuticals North America LLC under the name Jublia.
Definition
ChEBI: A member of the class of triazoles that is butan-2-ol which is substituted at positions 1, 2, and 3 by 1,2,4-triazol-1-yl, 2,4-difluorophenyl, and 4-methylenepiperidin-1-yl groups, respectively (the 2R,3R stereoisomer). It is an antifungal drug used for the topical treatment of onychomycosis (a nail infection caused mainly by dermatophytes).
brand name
Jublia
Biochem/physiol Actions
Efinaconazole is a triazole antifungal drug approved clinically for the treatment of nail fungus (Onychomycosis). It inhibits sterol biosynthesis by inhibition of cytochrome P450 14α-demethylase, an enzyme in the sterol biosynthesis pathway that leads from lanosterol to ergosterol. Efinaconazole has better nail penetration, so is more effective than other topical agents and as effective as oral medication for nail fungus.
Pharmacokinetics
mean ± SD plasma Cmax on Day 28 of treatment: 0.67 ± 0.37 ng/mL. mean ± SD AUC was 12.15 ± 6.91 ng*h/mL.
Synthesis
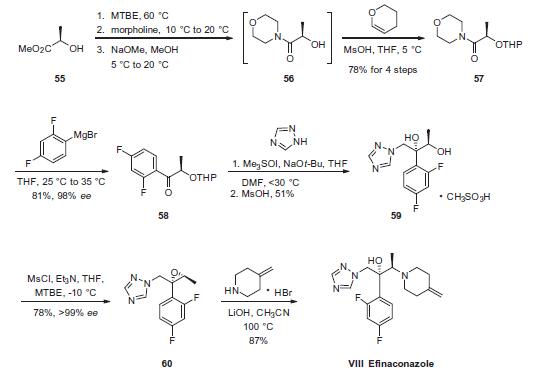
Commercially available (R)-methyl lactate (55) was first converted to THP protected alcohol 57 in 4 steps and 78% yield via morpholino amide 56. Grignard displacement of the morpholine afforded ketone 58 in 81% yield. Next, ketone 58 was epoxidized by means of the Corey ylide followed by ring-opening of the epoxide by triazole which had been activated by exposure to sodium tbutoxide. Finally, subjection to methanesulfonic acid furnished diol 59 in 51% yield as the corresponding mesylate salt. Diol 59 was then converted to epoxide 60 through the use of mesyl chloride and triethylamine in 78% yield and >99% ee. Finally, treatment of epoxide 60 with 4-methylene piperidine¨CHBr in the presence of lithium hydroxide afforded efinaconazole (VIII) in 87% yield.
Metabolism
Not Available
Properties of KP 103
| Melting point: | 192-195°C |
| Boiling point: | 512.2±60.0 °C(Predicted) |
| Density | 1.26±0.1 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | powder |
| pka | 12.11±0.29(Predicted) |
| color | white to beige |
| optical activity | [α]/D -85 to -95°, c = 1 in chloroform |
Safety information for KP 103
| Signal word | Warning |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H361:Reproductive toxicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for KP 103
KP 103 manufacturer
Virupaksha Organics Pvt Ltd
Solara Active Pharma Sciences Ltd
BDR Pharmaceuticals International Pvt Ltd
Turtle Pharma Private Limited
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 Efinaconazole 98%View Details
Efinaconazole 98%View Details -
 Efinaconazole 99%View Details
Efinaconazole 99%View Details
164650-44-6 -
 Efinaconazole 164650-44-6 98%View Details
Efinaconazole 164650-44-6 98%View Details
164650-44-6 -
 Efinaconazole 164650-44-6 98%View Details
Efinaconazole 164650-44-6 98%View Details
164650-44-6 -
 Efinaconazole 98%View Details
Efinaconazole 98%View Details -
 164650-44-6 Efinaconazole 98%View Details
164650-44-6 Efinaconazole 98%View Details
164650-44-6 -
 Efinaconazole 98% (HPLC) CAS 164650-44-6View Details
Efinaconazole 98% (HPLC) CAS 164650-44-6View Details
164650-44-6 -
 Efinaconazole CAS 164650-44-6View Details
Efinaconazole CAS 164650-44-6View Details
164650-44-6