Ravuconazole
- CAS NO.:170864-29-6
- Empirical Formula: C22H17F2N5OS
- Molecular Weight: 437.47
- MDL number: MFCD09837767
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-02-23 21:28:46
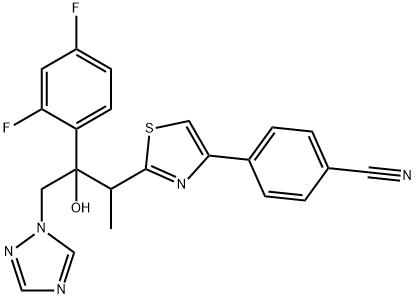
What is Ravuconazole?
Description
Ravuconazole belongs to a group of ‘‘second generation’’ triazoles; developed to overcome limitations, drug resistance, and drug interactions of the earlier triazoles. Formerly BMS-207147 and ER-30346, it was discovered by Eisai Co. Ltd. in Japan, developed by Bristol-Myers Squibb, and is now held again by Eisai. It is structurally related to fluconazole and voriconazole but, like isavuconazole, has a long half-life.
Chemical properties
Off-White Solid
The Uses of Ravuconazole
Ergosterol biosynthesis inhibitor. Antifungal.
Antimicrobial activity
The primary mode of action of ravuconazole is by inhibition of cytochrome P450 14a-demethylase, an enzyme in the sterol biosynthesis pathway. It is most potent against Candida spp. but has a broader spectrum of activity than fluconazole and itraconazole. It also has activity against Cryptococcus neoformans, Aspergillus fumigatus, dermatophytes, and dematiaceous fungi with limited activity against Sporothrix schenckii, Pseudallescheria boydii, Scedosporium apiospermum, Fusarium spp., and Zygomycetes. It is currently undergoing phase II clinical trials.
Biological Activity
In animal models, the oral bioavailability of ravuconazole ranged from 48% to 74%. The presence of food enhanced absorption, with a 2- to 4-fold increase in bioavailability when administered with a high-fat meal. Ravuconazole had a long serum elimination half-life ranging from 3.9 to 202 hours. Protein binding was high at 95.8% to 98%.
Mechanism of action
As with the other azoles, ravuconazole inhibits the P450-dependent enzyme lanosterol 14-a demethylase, resulting in depletion of ergosterol and the accumulation of 14-a demethylated precursors. This interferes with the function of ergosterol in fungal membranes and breaks down the integrity of the membranes.
Toxicology
For doses o2.5 mg/kg/day in human volunteers, there has been minimal hepatotoxicity and nephrotoxicity. Headache and abdominal pain were most frequently reported, followed by diarrhea, pruritus, and rash. No side-effects have been noted in rats and dogs treated with ravuconazole for a week. There are still ongoing clinical trials assessing the tolerability of this drug in both the oral and parenteral formulations.
Drug interactions
Ravuconazole may have a lower potential for drug interactions as it is a less-potent inhibitor of CYP3A4 than voriconazole; however, no information is available about interaction with other liver cytochrome enzymes such as CYP2C9 or 2C19. One clinical trial of ravuconazole in subjects with oral candidiasis and HIV found that rifampicin reduced ravuconazole levels by over 50%.
Properties of Ravuconazole
| Melting point: | 148-151°C |
| storage temp. | -20°C Freezer |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| color | Off-White |
Safety information for Ravuconazole
Computed Descriptors for Ravuconazole
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 1-methylindoline-2,3-dione 98%View Details
1-methylindoline-2,3-dione 98%View Details
2058-74-4 -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
