Luliconazole
- CAS NO.:187164-19-8
- Empirical Formula: C14H9Cl2N3S2
- Molecular Weight: 354.27
- MDL number: MFCD00953915
- EINECS: 247-960-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-11-22 16:52:42
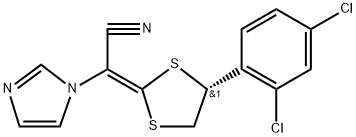
What is Luliconazole?
Absorption
Although luliconazole is administered topically, clinical studies have shown that after the first dose in patients with tina pedis, a maximum plasma concentration of 0.40 ± 0.76 ng/mL (mean ± SD) occurred in 16.9 ± 9.39 hours (mean ± SD).
Toxicity
In clinical trials, no serious toxicity was reported, only local irritation (mild contact dermatitis and cellulitis) at the site of application was found.
Description
Luliconazole is a member of the imidazole class of antifungal agents, with specific utility as a dermatological antimycotic drug. It was launched in Japan as a topical agent for the treatment of athlete’s foot. Luliconazole is an optically active drug with (R)-configuration at its chiral center. It is structurally related to lanoconazole, which has been marketed as a racemic mixture since 1994. As with other azole antifungal drugs, the mechanism of action of luliconazole is the inhibition of sterol 14-a-demethylase, and subsequently, inhibition of ergosterol biosynthesis. The in vivo activity of luliconazole against dermatophytes has been evaluated in the guinea pig model of tinea pedis. In this study, a 1% topical solution of luliconazole, administered once daily for seven days, achieved complete mycologic cure. Additionally, there were no occurrences of relapse for up to 16 weeks after the treatment. No data is currently available on the clinical efficacy of luliconazole. The chemical synthesis of luliconazole involves the condensation of 1-(cyanomethyl)imidazole with carbon disulfide to produce a dithioate intermediate, and subsequent alkylation with either the mesylate derivative of (S′)-1-(2,4-dichlorophenyl)-2-bromoethanol or the bis-mesylate derivative of (S′)-1-(2,4-dichlorophenyl)ethane-1,2-diol. .
Originator
Nihon Nohyaku (Japan)
The Uses of Luliconazole
Luliconazole is an azole antifungal drug. It was approved by the FDA (USA) in November 2013 and is marketed under the brand name Luzu. Luliconazole is also approved in Japan.
Indications
Luliconazole is indicated for the treatment of interdigital tinea pedis, tinea cruris, or tinea corporis infections caused by Trichophyton rubrum and Epidermophyton floccosum.
Background
Luliconazole is a topical antifungal agent that acts by unknown mechanisms but is postulated to involve altering the synthesis of fungi cell membranes. It was approved by the FDA (USA) in November 2013 and is marketed under the brand name Luzu. Luliconazole is also approved in Japan.
Definition
ChEBI: Luliconazole is a dichlorobenzene.
brand name
Lulicon
Pharmacokinetics
Luliconazole kills the organisms Trichophyton rubrum and Epidermophyton floccosum, most likely by altering their fungal cell membranes.
Synthesis
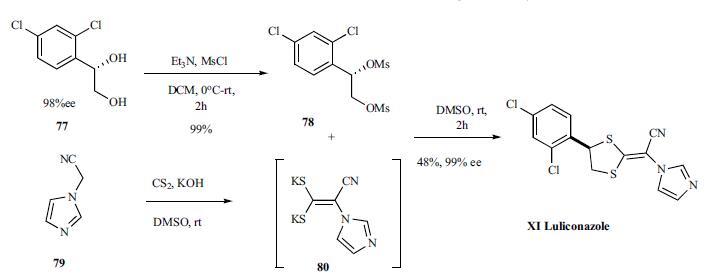
Synthesis of luliconazole started with diol 77, prepared according to literature procedure in 98%ee which was activated by converting to dimesylate 78 in 99% yield and coupled to dipotassium enolate 80, prepared in situ by reacting cyano methylimidazole 79 with carbon disulfide, to give luliconazole (XI), 99% ee in 48% yield.
Metabolism
The metabolism of luliconazole has yet to be determined.
Mode of action
The mechanism of action of luliconazole is as a Cytochrome P450 2C19 Inhibitor.
Properties of Luliconazole
| Melting point: | 152 °C |
| Boiling point: | 499.1±55.0 °C(Predicted) |
| Density | 1.52±0.1 g/cm3(Predicted) |
| storage temp. | Sealed in dry,Store in freezer, under -20°C |
| solubility | Chloroform (Slightly), Ethyl Acetate (Slightly) |
| form | Solid |
| pka | 3.76±0.10(Predicted) |
| color | White to Off-White |
| Stability: | Light Sensitive |
| CAS DataBase Reference | 187164-19-8 |
Safety information for Luliconazole
Computed Descriptors for Luliconazole
Luliconazole manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 Luliconazole 99%View Details
Luliconazole 99%View Details -
 Luliconazole 99%View Details
Luliconazole 99%View Details -
 Luliconazole 99%View Details
Luliconazole 99%View Details -
 187164-19-8 98%View Details
187164-19-8 98%View Details
187164-19-8 -
 Luliconazole 99%View Details
Luliconazole 99%View Details
187164-19-8 -
 LULICONAZOLE 95-99 %View Details
LULICONAZOLE 95-99 %View Details
187164-19-8 -
 Luliconazole 95% CAS 187164-19-8View Details
Luliconazole 95% CAS 187164-19-8View Details
187164-19-8 -
 Luliconazole Powder ApiView Details
Luliconazole Powder ApiView Details
187164-19-8
