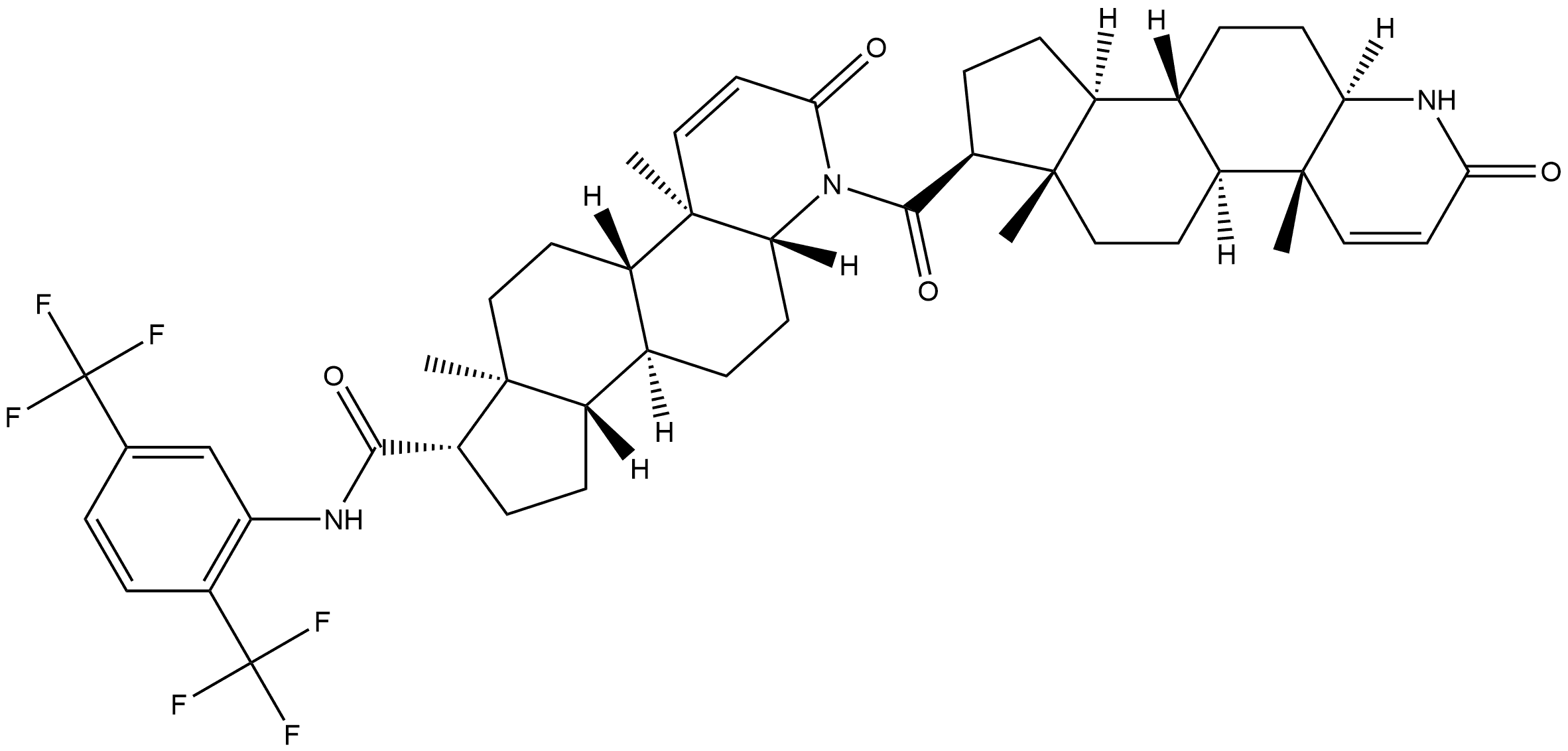
Dutasteride
Synonym(s):(5α,17β)-N-[2,5-Bis(trifluoromethyl)phenyl]-3-oxo-4-azaandrost-1-ene-17-carboxamide;17β-N-[2,5-Bis(trifluoromethyl)phenyl]carbamoyl-4-aza-5α-androst-1-en-3-one;Dutasteride
- CAS NO.:164656-23-9
- Empirical Formula: C27H30F6N2O2
- Molecular Weight: 528.53
- MDL number: MFCD00937869
- EINECS: 638-758-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-20 11:34:09

What is Dutasteride?
Absorption
Following oral administration of a single dose of 0.5 mg dutasteride, the peak serum concentrations were reached within 2 to 3 hours. Following daily oral administration of 0.5 mg dutasteride, the steady-state concentration of 40 ng/mL is expected to be achieved at 6 months following initial administration. In healthy subjects, the absolute bioavailability was 60%, ranging from 40% to 94%. While food intake reduced the maximum serum concentrations by 10 to 15%, food intake is reported to have a negligible effect on the bioavailability of the drug.
Toxicity
LD50 values
The estimated dermal LD50 of dutasteride in rabbits is > 2,000 mg/kg.
Overdose
In studies of volunteers receiving single doses of dutasteride up to 40 mg (which is 80 times the therapeutic dose) for 7 days, there were no reports of clinically significant adverse events. Low incidences of impotence, reduced libido, gynecomastia, and ejaculation disorder occurred significantly more often in dutasteride than placebo recipients. There are no known antidotes for dutasteride. In case of overdose, appropriate symptomatic and supportive treatment should be given.
Pregnancy and Lactation
As DHT is a necessary hormone for the development of male genitalia, exposure to dutasteride in pregnant women bearing male fetuses may cause fetal harm. In animal reproduction and developmental toxicity studies, dutasteride inhibited normal development of external genitalia in male fetuses. Although it is not known whether dutasteride is excreted in human milk, the use of dutasteride in women of childbearing potential, including nursing women. In elderly patients, the half-life of dutasteride may increase. As the renal elimination of dutasteride is very minimal, the use of dutasteride in patients renal insufficiency is reported to be safe. There are no specific dosage adjustment recommendations for use in elderly patients or patients with renal impairment.
Description
Dutasteride is a synthetic 4-azasteroid compound. It is a dual inhibitor of type 1 and 2 isoforms of 5α-reductase unlike finasteride, the first marketed 5α-reductase inhibitor, which only acts on type 2 isozyme. Dutasteride is a 3-fold greater inhibitor of type-2 5α-reductase than finasteride in men and has greater effect on the type-l than on type-2 isozyme. In animal models,dutasteride exhibited superior efficacy and pharmacokinetics compared to finasteride. In patients with benign prostate hyperplasia, administration of dutasteride was shown to dose-dependently decrease serum dihydrotestosterone levels with greater efficacy as compared to finasteride (95% vs 67%). Serum testosterone levels increased with both active drugs, in conjunction with dihydrotestosterone suppression but remained within normal ranges. In long term studies, in men with moderate to severe benign prostate hyperplasia, once daily dutasteride significantly reduced prostate volume, reduced the risk of acute urinary retention and surgery by 57% and improved lower urinary tract symptoms and urinary flow measurements.
Chemical properties
Dutasteride is a white to pale yellow powder It is soluble in ethanol (44 mg/mL), methanol (64 mg/mL), and polyethylene glycol 400 (3 mg/mL), but it is insoluble in water.
Originator
GlaxoSmithKline (UK)
The Uses of Dutasteride
Dutasteride is a dual inhibitor of 5a-reductase isoenzymes type 1 and 2; structurally related to Finasteride. Dutasteride is used in the treatment of benign prostatic hyperplasia. Dutasteride is associated with a low rate of transient serum aminotransferase elevations, but has yet to be linked to instances of clinically apparent acute liver injury.
Background
Dutasteride is an oral synthetic 4-azasteroid commonly marketed under the trade name Avodart. It is a novel dual 5α-reductase inhibitor that works by blocking both isoforms of 5α-reductase enzymes in a potent, selective, and irreversible manner. Type I and II 5α-reductase enzymes convert testosterone into dihydrotestosterone (DHT), a primary hormonal mediator that plays a role in the development and enlargement of the prostate gland. Dutasteride was approved by the FDA in 2001 for the treatment of symptomatic benign prostatic hyperplasia (BPH) in men as monotherapy or in combination with the α-adrenergic antagonist tamsulosin to enhance the therapeutic response. Its clinical efficacy against benign prostate hyperplasia in male patients is comparable to that of finasteride, a specific type II 5α-reductase inhibitor. However, unlike finasteride, dutasteride is not yet indicated for the treatment of androgenic alopecia although it was demonstrated to be effective in several randomized, double-blind, placebo-controlled trials in androgenetic alopecia.
Indications
Indicated for the treatment of symptomatic benign prostatic hyperplasia (BPH) in men with an enlarged prostate gland to improve symptoms, and reduce the risk of acute urinary retention and the need for BPH-related surgery alone or in combination with tamsulosin.
Definition
ChEBI: Dutasteride is an aza-steroid that is inasteride in which the tert-butyl group is replaced by a 2,5-bis(trifluoromethyl)phenyl group. A synthetic 4-azasteroid, dutasteride is a selective inhibitor of both the type 1 and type 2 isoforms of steroid 5alpha-reductase, an intracellular enzyme that converts testosterone to 5alpha-dihydrotestosterone. Dutasteride is used for the treatment of symptomatic benign prostatic hyperplasia in men with an enlarged prostate gland. It has a role as an EC 1.3.1.22 [3-oxo-5alpha-steroid 4-dehydrogenase (NADP(+))] inhibitor and an antihyperplasia drug. It is an aza-steroid, a member of (trifluoromethyl)benzenes and a delta-lactam. It derives from a hydride of a 5alpha-androstane.
brand name
Avodart (GlaxoSmithKline).
Biological Functions
Similar to finasteride, dutasteride is a competitive and mechanism-based inhibitor not only of type 2 but also of type 1 5α-reductase isoenzymes, with which stable enzyme-NADP adduct complexes are formed, inhibiting the conversion of testosterone to DHT. The suppression of both type 1 and type 2 isoforms results in greater and more consistent reduction of plasma DHT than that observed for finasteride. The more effective dual inhibition of type 1 and type 2 5α-reductase isoforms lowers circulating DHT to a greater extent than with finasteride and shows advantages in treating BPH and other disease states (e.g., prostate cancer) that are DHT-dependent.
Biochem/physiol Actions
Dutasteride is a potent dual inhibitor of 5α-reductase isoenzymes types 1 and 2 (IC50 = 6 nM 5-AR1; 7 nM 5-AR2). Dutasteride blocks testosterone conversion to dihydrotesterone, and is used clinically for treating benign prostatic hyperplasia (BPH).
Pharmacokinetics
Dutasteride is a synthetic 4-azasteroid compound that selectively inhibits both the type I and type II isoforms of steroid 5α-reductase, an intracellular enzyme that converts testosterone to 5α-dihydrotestosterone (DHT). Dutasteride works by reducing the levels of circulating DHT. It was also shown to reduce the size of the prostate gland, improve urinary flow, and symptoms of benign prostatic hyperplasia alone or in combination with tamsulosin. The effect of the reduction of DHT by dutasteride is dose-dependent, with the maximum effect observed within 1-2 weeks following initial administration.
After 1 and 2 weeks of daily dosing with dutasteride 0.5 mg, median serum DHT concentrations were reduced by 85% and 90%, respectively. The serum concentrations of DHT were maintained to be decreased by more than 90% in 85% of patients following 1 years' administration of oral dutasteride 0.5 mg/day. As evident from the clinical studies, dutasteride may also cause decreases in serum PSA in the presence of prostate cancer.
Pharmacokinetics
The maximum effect of 0.5 mg daily doses of dutasteride on the suppression of DHT is dose-dependent and is observed within 1 to 2 weeks. After 2 weeks of 0.5 mg daily dosing, median plasma DHT concentrations were reduced by 90%, and after 1 year, the median decrease in plasma DHT was 94%. The median increase in plasma testosterone was 19% but remained within the physiological range. The drug also reduced serum prostatic specific antigen by approximately 50% at 6 months and total prostate volume by 25% at 2 years. Dutasteride produced improvements in quality of life and peak urinary flow rate and reduction of acute urinary retention without the need for surgery.
Clinical Use
Dutasteride belongs to azasteriod class of compounds and function as a 5α-reductase inhibitor1 which prevents the conversion of the androgen sex hormone testosterone into the more potent metabolite dihydrotestosterone (DHT). In 2009, South Korea has been licensed dutasteride for the treatment of androgenetic alopecia and in Japan 2015.
Dutasteride is the first and only double 5α reductase inhibitor used to treat Benign prostatic hyperplasia, and it is mainly used clinically to treat prostate enlargement, male-pattern hair loss, seborrheic hair loss, and hereditary hair loss.
Side Effects
The main side effects are ED, decreased libido, gynecomastia, and ejaculation disorders. Long-term use (>4 years), however, did not reveal increased onset of sexual side effects. In addition, the combination of dutasteride and tamsulosin is well-tolerated and has the added advantage of rapid symptomatic relief.
Synthesis
Dutasteride can be prepared from 3-oxo-4-androstene-17β-carboxylic acid by several ways in 6 or 8 steps. In the preparation of dutasteride, the introduction of the carbon-carbon double bond in conjugation with C-3 carbonyl carbon of azaandrosteriods is one of the most important chemical reaction.
an efficient synthesis of dutasteride: utilizing benzoyl group as novel lactamic protecting group
in vivo
dutasteride, which inhibits both 5αr1/5αr2, is efficacious in blocking prostate cancer development or progression in c57bl/6 tramp x fvb mice [2].
Metabolism
Dutasteride undergoes extensive hepatic metabolism mediated by CYP3A4 and CYP3A5. 4′-hydroxydutasteride, 6-hydroxydutasteride, 6,4′-dihydroxydutasteride, 1,2-dihydrodutasteride, and 15-hydroxydutasteride metabolites are formed. 2 minor metabolites - 6,4′-dihydroxydutasteride and 15-hydroxydutasteride - can also be detected. According to in vitro studies, 4′-hydroxydutasteride and 1,2-dihydrodutasteride mediated inhibitory actions against both isoforms of 5α-reductase but with lower potency when compared to the parent drug. The activity of 6β-hydroxydutasteride is comparable to that of dutasteride.
Metabolism
Dutasteride is metabolised by the cytochrome P450 isoenzymes CYP3A4 and CYP3A5, and most of a dose is excreted as metabolites in the faeces.
Side Effects
Sexual problems (such as decreased sexual interest/ability, decrease in the amount of semen/sperm), testicle pain/swelling, increased breast size, or breast tenderness may occur. Sexual problems have continued in some men even after stopping treatment.
Mode of action
The human body contains type I and type II 5α reductase, with type II found mainly in the prostate, and type I found mainly in the liver and skin. 5α reductase is the main cause for continuous benign prostate enlargement; it promotes the transformation of testosterone in patients’ prostate into the more active dihydrotestosterone, thus causing prostate cells to enlarge and the prostate to swell. Dutasteride can inhibit both type I and II 5α reductase at the same time. This type of simultaneous inhibiting mechanism can rapidly and continuously reduce prostate size, dramatically improve urination, and reduce the risk fo acute urinary retention and its related prostate surgeries.
Clinical claims and research
The American FDA approved a 2-year multicenter randomized double-blind control clinical trial – the first long term clinical assessment of the combined usage of Dutasteride and α receptor blockers. Included subjects were male patients with moderate to severe prostate enlargement (ages greater than or equal to 50, prostate volume (PV) ≥30 cc, serum prostate specific antigen (PSA) levels 1.5-10ng/ml, 5ml/sec < maximum urinary flow (Qmax) ≤15ml/sec, minimum urination ≥ 125ml, international prostate symptom score (IPSS) ≥ 12). Patients were first given a placebo for 4 weeks and then were randomly given either 0.5mg/day of Dutasteride and 0.4mg/day of Tamsulosin, only 0.5mg/day of Dutasteride, or only 0.4mg/day of Tamsulosin.
Results showed: After 12-24 months, the combined usage of Dutasteride with Tamsulosin had better curative effects than did individual usage.
References
[1] schmidt lj1, murillo h, tindall dj. gene expression in prostate cancer cells treated with the dual 5 alpha-reductase inhibitor dutasteride. j androl. 2004 nov-dec;25(6):944-53.
[2] opoku-acheampong ab1, unis d, henningson jn, beck ap, lindshield bl.preventive and therapeutic efficacy of finasteride and dutasteride in tramp mice. plos one. 2013 oct 18;8(10):e77738. doi: 10.1371/journal.pone.0077738. ecollection 2013.
Properties of Dutasteride
| Melting point: | 242-250°C |
| Boiling point: | 620.3±55.0 °C(Predicted) |
| Density | 1.303±0.06 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | DMSO: soluble2mg/mL, clear |
| pka | 13.32±0.70(Predicted) |
| form | powder |
| color | white to beige |
| optical activity | [α]/D +18 to +24°, c = 1 in chloroform-d |
Safety information for Dutasteride
| Signal word | Danger |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H351:Carcinogenicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for Dutasteride
| InChIKey | JWJOTENAMICLJG-QWBYCMEYSA-N |
| SMILES | N1[C@@]2([H])[C@@](C)([C@@]3([H])CC[C@@]4(C)[C@]([H])([C@]3([H])CC2)CC[C@@H]4C(NC2=CC(C(F)(F)F)=CC=C2C(F)(F)F)=O)C=CC1=O |
Dutasteride manufacturer
Stermone Chemicals Pvt Ltd
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 164656-23-9 98%View Details
164656-23-9 98%View Details
164656-23-9 -
 Dutasteride 98%View Details
Dutasteride 98%View Details
164656-23-9 -
 Dutasteride 99%View Details
Dutasteride 99%View Details -
 Dutasteride 99%View Details
Dutasteride 99%View Details -
 Dutasteride 99%View Details
Dutasteride 99%View Details -
 DUTASTERIDE 99%View Details
DUTASTERIDE 99%View Details -
 Dutasteride >98% (HPLC) CAS 164656-23-9View Details
Dutasteride >98% (HPLC) CAS 164656-23-9View Details
164656-23-9 -
 Dutasteride 95.00% CAS 164656-23-9View Details
Dutasteride 95.00% CAS 164656-23-9View Details
164656-23-9