Elagolix Sodium
- CAS NO.:832720-36-2
- Empirical Formula: C32H29F5N3NaO5
- Molecular Weight: 653.571546
- MDL number: MFCD12546649
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 11:34:44
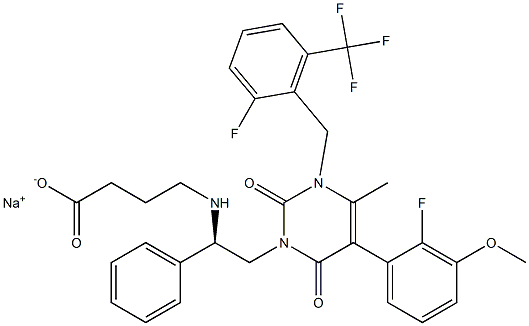
What is Elagolix Sodium?
Description
Elagolix sodium (ABT-620) is a small molecule, orally active gonadotropin-releasing hormone (GnRH) receptor antagonist. It has the ability to suppress estradiol (E2) concentrations in a dose-dependent manner.
It was originally developed by Neurocrine Biosciences, then licensed to Abbott (now Abbvie) for worldwide co-development of this indication in 2010. AbbVie is applying to use elagolix for the treatment of endometriosis with associated pain. Elagolix is the first orally available GnRH antagonist, and as such may provide patients with relief from the painful symptoms of endometriosis while offering advantages over currently available injectable peptide-based treatments. The estimated patient population is over 170 million women worldwide.
Description
Elagolix is an antagonist of the gonadotropin-releasing hormone receptor (GnRHR; Ki = 0.9 nM in a radioligand binding assay). It is selective for GnRHR over the cytochrome P450 (CYP) isoform CYP3A4 (IC50 = 56 μM) as well as a panel of 100 receptors, ion channels, enzymes, and transporters (IC50s = >10 μM). Elagolix inhibits GnRH-induced inositol phosphate production in RBL-1 cells expressing human GnRHR (IC50 = 1.5 nM). In vivo, elagolix (30 mg/kg) suppresses production of luteinizing hormone in castrated male cynomolgus macaques. Formulations containing elagolix have been used in the treatment of endometriosis.
Chemical properties
Elagolix sodium is a small synthetic molecule chemically known as Sodium 4-({(1R)-2- [5-(2-fluoro-3-methoxyphenyl)-3-{[2-fluoro-6-(trifluoromethyl) phenyl]methyl}-4- methyl -2,6-dioxo-3,6-dihydropyrimidin-1(2H)-yl]-1-phenylethyl} amino)butanoate. The structure was confirmed by mass spectrometry, infrared spectroscopy, nuclear magnetic resonance.
Elagolix sodium is isolated as a hygroscopic, amorphous white to off-white to light yellow powder.
The Uses of Elagolix Sodium
Elagolix Sodium is a gonadotropin releasing hormone antagonist (GnRH antagonist) used in the treatment of pain associated with endometriosis in women. It is also in phase III clinical trials for the treatment of uterine fibroids in women. Endometriosis is a frequent cause of infertility, connected with a chronic pelvic and pre-menstrual pain.
Elagolix sodium for the treatment of women with moderate to severe endometriosis-associated pain
The Uses of Elagolix Sodium
Elagolix Sodium is a novel uracil phenylethylamine that acts as potent human gonadotropin-?releasing hormone receptor (hGnRH-?R) antagonist.
Synthesis Reference(s)
PROCESS FOR THE PREPARATION OF ELAGOLIX SODIUM AND ITS POLYMORPH
The US patent number 7056927 B2 discloses, elagolix sodium salt as a white solid and process for its preparation in Example-1; Step-IH.
The US patent number 8765948 B2 discloses a process for preparation of amorphous elagolix sodium by spray drying method and solid dispersion of amorphous elagolix sodium with a polymer.
in vitro
Elagolix sodium is a human GnRH receptor (GnRHR) antagonist with an IC50 of 0.25 nM in Kinase assay. Elagolix sodium has advanced to phase 3 trials for the treatment of endometriosis and uterine fibroids. Elagolix sodium also shows NFAT inhibition with an IC50 of 5.4 nM and effectively blocks Ca2+ flux with an IC50 of 0.86 nM[1]. Kinase assay also demonstrates that Elagolix sodium is a human GnRH receptor (GnRHR) antagonist with a Ki value of 3.7 nM[2].
Properties of Elagolix Sodium
| Melting point: | >188°C (dec.) |
| storage temp. | Hygroscopic, -20°C Freezer, Under inert atmosphere |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| color | White to Off-White |
| Stability: | Hygroscopic |
Safety information for Elagolix Sodium
Computed Descriptors for Elagolix Sodium
| InChIKey | DQYGXRQUFSRDCH-YEZAQYPZNA-M |
| SMILES | [Na+].C([O-])(=O)CCCN[C@H](C1=CC=CC=C1)CN1C(=O)N(CC2=C(C(F)(F)F)C=CC=C2F)C(C)=C(C2=CC=CC(OC)=C2F)C1=O |&1:8,r| |
Elagolix Sodium manufacturer
LUPA PHARMACEUTICALS
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
![1-[2-fluoro-6-(trifluoromethyl)benzyl]-5-iodo-6-methylpyrimidine-2,4(1H,3H)-dione](https://img.chemicalbook.in/CAS/GIF/1150560-54-5.gif)

You may like
-
 832720-36-2 Elagolix Sodium 98%View Details
832720-36-2 Elagolix Sodium 98%View Details
832720-36-2 -
 Elagolix sodium 98%View Details
Elagolix sodium 98%View Details -
 Elagolix sodium 98%View Details
Elagolix sodium 98%View Details -
 832720-36-2 ELAGOLIX SODIUM 95-99 %View Details
832720-36-2 ELAGOLIX SODIUM 95-99 %View Details
832720-36-2 -
 832720-36-2 Elagolix Sodium 98+View Details
832720-36-2 Elagolix Sodium 98+View Details
832720-36-2 -
 Elagolix sodium 95% CAS 832720-36-2View Details
Elagolix sodium 95% CAS 832720-36-2View Details
832720-36-2 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
